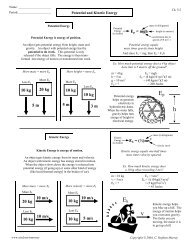
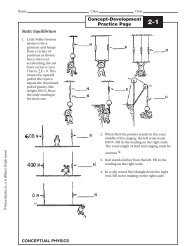
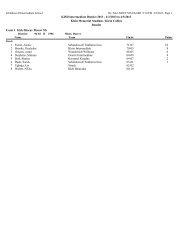
Exercise 1
Exercise 1
Exercise 1
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Stoichiometry9. Calculate the number of grams of carbon dioxide which will be formed inthe reaction between 10.0 grams of propane (C 3 H 8 ) and 10.0 grams ofoxygen.10. In the reaction single replacement reaction between gold III sulfide andhydrogen gas if the initial amount of Au 2 S 3 is 500.20 g and the initialamount of H 2 is 5.67g, what is the final mass of gold?11. In a reaction between magnesium nitride and water, magnesiumhydroxide and ammonia (NH3) are produced. If the initial amount ofMg 3 N 2 is 58.1g and the initial amount of H 2 O is 20.4g, what is the finalmass of ammonia?12. In the reaction 2C 3 H 6 + 2NH 3 + 3O 2 2C 3 H 3 N + 6H 2 O, if the initialamounts are C 3 H 6 : 22.5g, NH 3 : 20.6 g, and O 2 : 18.1 g, what are the finalamounts of each product?13. What is the limiting reactant when 19.9g of CuO are exposed to 2.02g ofH 2 according to the following equation?CuO(s) + H 2 (g) Cu(s) + H 2 (g)How many grams of Cu are produced?14. Metallic magnesium reacts with steam to produce magnesium hydrociseand hydrogen ga. If 16.2 g of Mg are heated with 12.0 g of H 2 O, what isthe limiting reactant? How many grams of each product will beproduced?10-12HC/CC/TG KHS