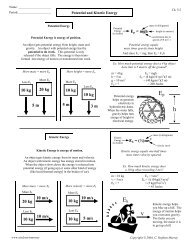
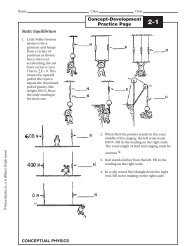
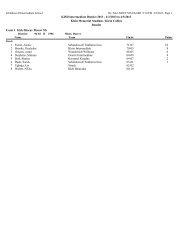
Exercise 1
Exercise 1
Exercise 1
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Stoichiometry<strong>Exercise</strong> 4H 3 PO 4 + MgCO 3 Mg 3 (PO 4 ) 2 + CO 2 + H 2 O1. Aluminum and iron (III) oxide react according to the following equationAl + Fe 2 0 3 - > A1 2 0 3 + FeHow much iron would be produced from 52.5g of iron (III) oxide?2. Ammonia is produced according to the following equation:N 2 + H 2 NH 3How many kilograms of nitrogen would be required to produce 556kilogram5 of ammonia?3. Calcium carbide is made in an electric furnace according to theecuation:CaO + C CaC 2 + CO75.5 kg of CaO will produce how many kilograms of calcium carbide?4. In the equation Fe + H 2 O Fe 2 O 3 + H 2235g of iron will produce how many grams of iron (III) oxide?5. Calcium chloride and water react according to the following equationCaCl 2 + H 2 O CaO + HC1How much hydrogen chloride would be produced from 37 4g of calciumchloride?6. how many grams. of iron (ii) bromide will be: neeced to react with anexcess of potassium phosphate to produce 17.1g of solid iron(ii)phosphate?7. How many grams of oxygen gas are required to react with 37.8g of solidzinc iodide, to produce solid iodine and solid zinc oxide?8. How many grams of silver can be produced by the reaction 5.05g ofsilver nitrate with excess copper?10-4HC/CC/TG KHS