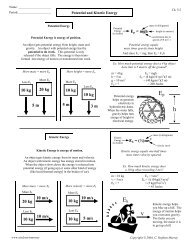
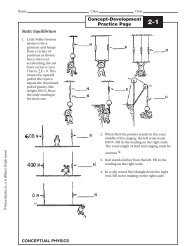
Exercise 1
Exercise 1
Exercise 1
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Stoichiometry9. What mass of barium chloride will be needed to react completely with113g of aluminum sulfate to produce barium sulfate and aluminum chloride?10. What mass of carbon dioxide will be produced by the combustion of40.09 of pentane, C 5 H 12 ?11. How many grams of copper (II) nitrate would be produced from 4.30g ofcopper metal reacting with excess hydrogen nitrate?12. When copper (II) nitrate reacts with sodium hydroxide, copper (II)hydroxide is produced along with sodium nitrate. How many grams ofcopper (II) hydroxide can! be prepared from 12.7g of copper (II) nitrate andexcess sodium hydroxide?13. When copper (II) hydroxide is heated it decomposes to black copper (II)oxide and water. How many grams of copper (II) oxide will be formed fromthe decomposition of 6.99g of copper(II) hydroxide?14. When 5.37g of the black copper(II) oxide is mixed with excess hydrogensulfate, the solution turns a clear blue, indicating the formation of copper (I)sulfate. How many grams of copper (II) sulfate will be formed in this doubledisplacement reaction?15. If an excess of zinc metal is placed in a copper (II) sulfate solution, thezinc will displace the copper. if 10.8g of copper(II) sulfate are reacted, howmany grams of copper metal will be recovered from the solution?10-5HC/CC/TG KHS