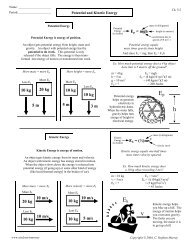
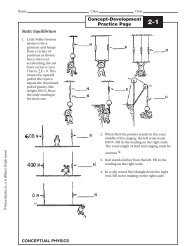
Exercise 1
Exercise 1
Exercise 1
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Stoichiometry<strong>Exercise</strong> 8Show your work.Indicate if the following are endothermic or exothermic:1. C + H 2 0 C0 2 + 2H 2 0 - 163 kJ2. C+ 1/20 2 - 121 kJ CO3. CO+ 1/20 2 CO 2 +283 kJ4. N 2 0 4 + 58 kJ 2NO 25. Given the following balanced equation:H 2 + F 2 ---> H 2 F 2 + 5.27 x 10 5 Ja. Is the reaction exothermic or endothermic?b. Calculate the number of joules of heat energy produced in the reaction of37.0 g of fluorine gas with sufficient hydrogen gas.6. Given the following balanced equation:O 2 + 2F 2 ---> 2OF 2 - 4.60 x 10 4 Ja. Is the reaction exothermic or endothermic?b. Calculate the number of grams of fluorine gas needed for the reactionwith 8.74 x 10 3 J of heat energy and sufficient oxygen gas.10-15HC/CC/TG KHS