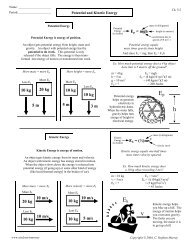
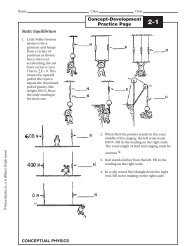
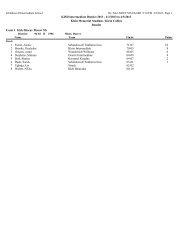
Exercise 1
Exercise 1
Exercise 1
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
StoichiometryQUESTIONS 1,2,3:REFER TO THE FOLLOWING EQUATION:Cu + HNO 3 Cu(NO 3 ) 2 + NO + H 2 O1. If you could drop 12 atoms of copper into a beaker containing hydrogennitrate, how many molecules of nitrogen dioxide would be produced?2. Calculate the number of moles of water produced when 6.6 molesof copper (II) nitrate are formed on the reaction.3. How many grams of copper would be needed to react with 4 moles ofhydrogen nitrate?4. 25g of potassium chlorate is to be decomposed by heating. How manygrams of oxygen can be prepared? KClO 3 ---> KC1 + O 25. In a reaction between sulfur and oxygen, 80g of sulfur dioxide is formed.How many grams of sulfur were burned?6. How many grams of hydrogen are required to completely convert 25g ofhot magnetic iron (III) oxide (Fe 2 O 3 ) to elementary iron?Fe 2 O 3 + H 2 Fe + H 2 O7. How many grams of chlorine will it take to react with 68g of potassiumiodide?8. Approximately 130g of zinc was dropped into a solution containingHC1. How many moles of hydrogen were produced?Zn + HC1 ZnC1 2 + H 29. Suppose 10g of iron (II) sulfide is treated with enough hydrogen chlorideto complete the reaction. How many grams of hydrogen sulfide gas couldbe collected? FeS + HC1 FeC1 2 + H 2 S10-9HC/CC/TG KHS