Notes of practical application of ion exchange resins in ... - Purolite
Notes of practical application of ion exchange resins in ... - Purolite
Notes of practical application of ion exchange resins in ... - Purolite
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
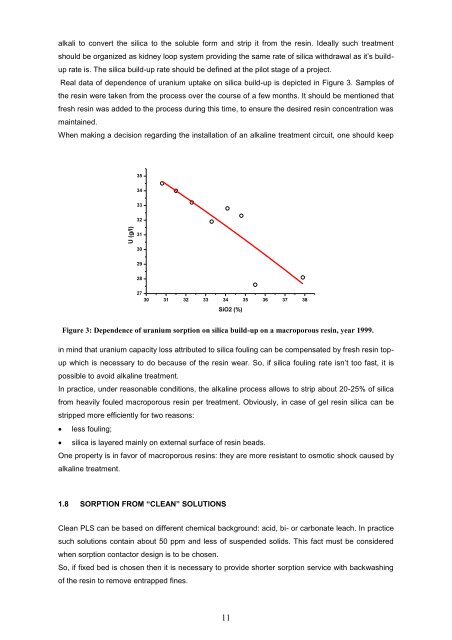
U (g/l)alkali to convert the silica to the soluble form and strip it from the res<strong>in</strong>. Ideally such treatmentshould be organized as kidney loop system provid<strong>in</strong>g the same rate <strong>of</strong> silica withdrawal as it’s builduprate is. The silica build-up rate should be def<strong>in</strong>ed at the pilot stage <strong>of</strong> a project.Real data <strong>of</strong> dependence <strong>of</strong> uranium uptake on silica build-up is depicted <strong>in</strong> Figure 3. Samples <strong>of</strong>the res<strong>in</strong> were taken from the process over the course <strong>of</strong> a few months. It should be ment<strong>ion</strong>ed thatfresh res<strong>in</strong> was added to the process dur<strong>in</strong>g this time, to ensure the desired res<strong>in</strong> concentrat<strong>ion</strong> wasma<strong>in</strong>ta<strong>in</strong>ed.When mak<strong>in</strong>g a decis<strong>ion</strong> regard<strong>in</strong>g the <strong>in</strong>stallat<strong>ion</strong> <strong>of</strong> an alkal<strong>in</strong>e treatment circuit, one should keep35343332313029282730 31 32 33 34 35 36 37 38SiO2 (%)Figure 3: Dependence <strong>of</strong> uranium sorpt<strong>ion</strong> on silica build-up on a macroporous res<strong>in</strong>, year 1999.<strong>in</strong> m<strong>in</strong>d that uranium capacity loss attributed to silica foul<strong>in</strong>g can be compensated by fresh res<strong>in</strong> topupwhich is necessary to do because <strong>of</strong> the res<strong>in</strong> wear. So, if silica foul<strong>in</strong>g rate isn’t too fast, it ispossible to avoid alkal<strong>in</strong>e treatment.In practice, under reasonable condit<strong>ion</strong>s, the alkal<strong>in</strong>e process allows to strip about 20-25% <strong>of</strong> silicafrom heavily fouled macroporous res<strong>in</strong> per treatment. Obviously, <strong>in</strong> case <strong>of</strong> gel res<strong>in</strong> silica can bestripped more efficiently for two reasons: less foul<strong>in</strong>g; silica is layered ma<strong>in</strong>ly on external surface <strong>of</strong> res<strong>in</strong> beads.One property is <strong>in</strong> favor <strong>of</strong> macroporous <strong>res<strong>in</strong>s</strong>: they are more resistant to osmotic shock caused byalkal<strong>in</strong>e treatment.1.8 SORPTION FROM “CLEAN” SOLUTIONSClean PLS can be based on different chemical background: acid, bi- or carbonate leach. In practicesuch solut<strong>ion</strong>s conta<strong>in</strong> about 50 ppm and less <strong>of</strong> suspended solids. This fact must be consideredwhen sorpt<strong>ion</strong> contactor design is to be chosen.So, if fixed bed is chosen then it is necessary to provide shorter sorpt<strong>ion</strong> service with backwash<strong>in</strong>g<strong>of</strong> the res<strong>in</strong> to remove entrapped f<strong>in</strong>es.11