Notes of practical application of ion exchange resins in ... - Purolite
Notes of practical application of ion exchange resins in ... - Purolite
Notes of practical application of ion exchange resins in ... - Purolite
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
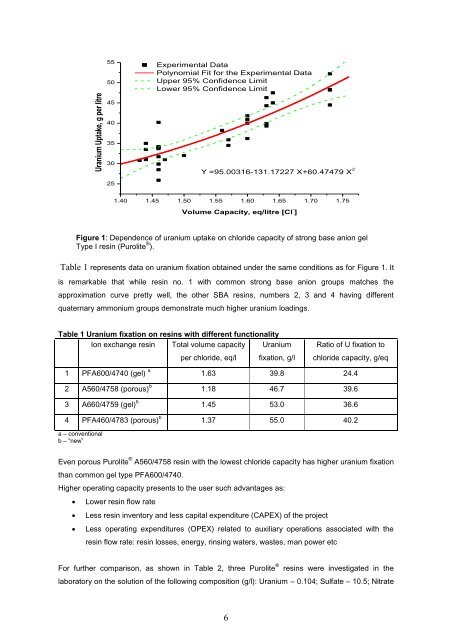
Uranium Uptake, g per litre5550Experimental DataPolynomial Fit for the Experimental DataUpper 95% Confidence LimitLower 95% Confidence Limit45403530Y =95.00316-131.17227 X+60.47479 X 2251.40 1.45 1.50 1.55 1.60 1.65 1.70 1.75Volume Capacity, eq/litre [Cl - ]Figure 1: Dependence <strong>of</strong> uranium uptake on chloride capacity <strong>of</strong> strong base an<strong>ion</strong> gelType I res<strong>in</strong> (<strong>Purolite</strong> ® ).Table 1 represents data on uranium fixat<strong>ion</strong> obta<strong>in</strong>ed under the same condit<strong>ion</strong>s as for Figure 1. Itis remarkable that while res<strong>in</strong> no. 1 with common strong base an<strong>ion</strong> groups matches theapproximat<strong>ion</strong> curve pretty well, the other SBA <strong>res<strong>in</strong>s</strong>, numbers 2, 3 and 4 hav<strong>in</strong>g differentquaternary ammonium groups demonstrate much higher uranium load<strong>in</strong>gs.Table 1 Uranium fixat<strong>ion</strong> on <strong>res<strong>in</strong>s</strong> with different funct<strong>ion</strong>alityIon <strong>exchange</strong> res<strong>in</strong> Total volume capacityper chloride, eq/lUraniumfixat<strong>ion</strong>, g/lRatio <strong>of</strong> U fixat<strong>ion</strong> tochloride capacity, g/eq1 PFA600/4740 (gel) a 1.63 39.8 24.42 A560/4758 (porous) b 1.18 46.7 39.63 A660/4759 (gel) b 1.45 53.0 36.64 PFA460/4783 (porous) b 1.37 55.0 40.2a – convent<strong>ion</strong>alb – “new”Even porous <strong>Purolite</strong> ® A560/4758 res<strong>in</strong> with the lowest chloride capacity has higher uranium fixat<strong>ion</strong>than common gel type PFA600/4740.Higher operat<strong>in</strong>g capacity presents to the user such advantages as: Lower res<strong>in</strong> flow rate Less res<strong>in</strong> <strong>in</strong>ventory and less capital expenditure (CAPEX) <strong>of</strong> the project Less operat<strong>in</strong>g expenditures (OPEX) related to auxiliary operat<strong>ion</strong>s associated with theres<strong>in</strong> flow rate: res<strong>in</strong> losses, energy, r<strong>in</strong>s<strong>in</strong>g waters, wastes, man power etcFor further comparison, as shown <strong>in</strong> Table 2, three <strong>Purolite</strong> ®<strong>res<strong>in</strong>s</strong> were <strong>in</strong>vestigated <strong>in</strong> thelaboratory on the solut<strong>ion</strong> <strong>of</strong> the follow<strong>in</strong>g composit<strong>ion</strong> (g/l): Uranium – 0.104; Sulfate – 10.5; Nitrate6