Chemistry 417-01 Inorganic Chemistry Exam 2 October 29, 2001 ...
Chemistry 417-01 Inorganic Chemistry Exam 2 October 29, 2001 ...
Chemistry 417-01 Inorganic Chemistry Exam 2 October 29, 2001 ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
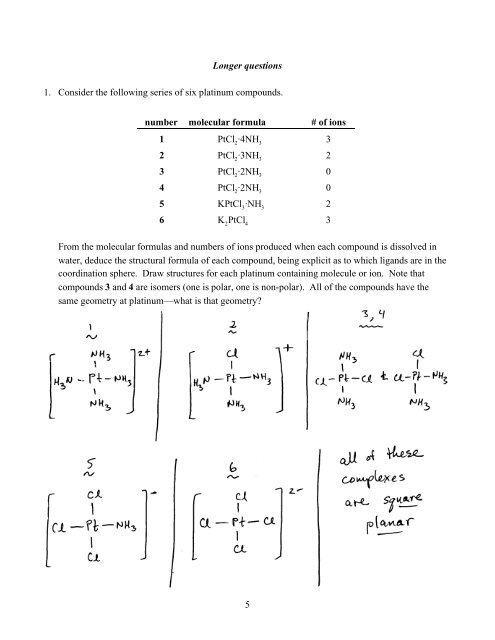
Longer questions1. Consider the following series of six platinum compounds.number molecular formula # of ions1 PtCl 2·4NH 332 PtCl 2·3NH 323 PtCl 2·2NH 304 PtCl 2·2NH 305 KPtCl 3·NH 326 K 2PtCl 43From the molecular formulas and numbers of ions produced when each compound is dissolved inwater, deduce the structural formula of each compound, being explicit as to which ligands are in thecoordination sphere. Draw structures for each platinum containing molecule or ion. Note thatcompounds 3 and 4 are isomers (one is polar, one is non-polar). All of the compounds have thesame geometry at platinum—what is that geometry?5