Chemistry 417-01 Inorganic Chemistry Exam 2 October 29, 2001 ...
Chemistry 417-01 Inorganic Chemistry Exam 2 October 29, 2001 ...
Chemistry 417-01 Inorganic Chemistry Exam 2 October 29, 2001 ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
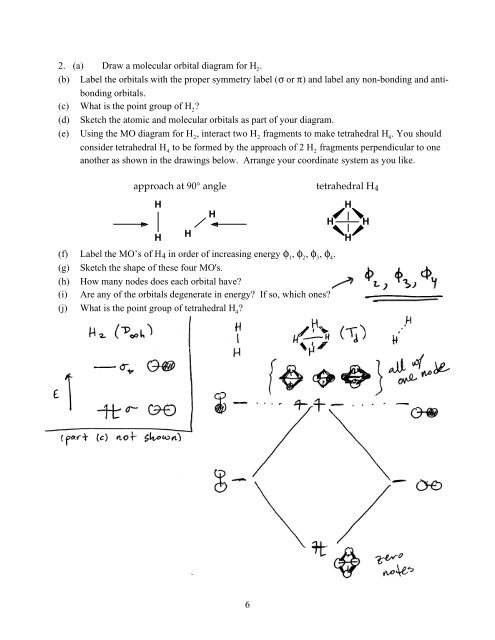
2. (a) Draw a molecular orbital diagram for H 2.(b) Label the orbitals with the proper symmetry label (σ or π) and label any non-bonding and antibondingorbitals.(c) What is the point group of H 2?(d) Sketch the atomic and molecular orbitals as part of your diagram.(e) Using the MO diagram for H 2, interact two H 2fragments to make tetrahedral H 4. You shouldconsider tetrahedral H 4to be formed by the approach of 2 H 2fragments perpendicular to oneanother as shown in the drawings below. Arrange your coordinate system as you like.approach at 90° angletetrahedral H 4HHH(f) Label the MO’s of H 4 in order of increasing energy φ 1, φ 2, φ 3, φ 4.(g) Sketch the shape of these four MO's.(h) How many nodes does each orbital have?(i) Are any of the orbitals degenerate in energy? If so, which ones?(j) What is the point group of tetrahedral H 4?HHHHH6