Kinetic Isotope Effect
Kinetic Isotope Effect
Kinetic Isotope Effect
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
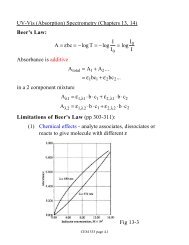
Chemistry 472 Fall 2000Rate Constant Determination and <strong>Kinetic</strong> <strong>Isotope</strong> <strong>Effect</strong>sIntroductionIn this experiment the spontaneous hydrolysis of acetic anhydride in water is studied using two methods:UV-Vis spectroscopy and solution conductance measurements. Rate constants are determined and theproposed rate law is evaluated. Additionally, information about the rate determining step is elucidated bycomparing the reaction rates in H 2 O and D 2 O. Useful references include: the “Conductance of Solutions”in Experiments in Physical Chemistry by Shoemaker, Garland, and Nibler; “<strong>Isotope</strong> <strong>Effect</strong>s” inMechanisms and Theory in Organic Chemistry by Lowry and Richardson or Advanced OrganicChemistry by Casey and Sundberg; Principles of Instrumental Analysis by Skoog, Holler, and Nieman;and your physical chemistry textbook.Reaction rates are determined by following the change in concentration of reactants and/or products as thereaction proceeds. The technique chosen for monitoring concentration changes depends upon thephysical properties of the compounds in the reaction mixture. In this experiment, the spontaneoushydrolysis of acetic anhydride to form acetic acid is studied:(CH 3 CO) 2 O + H 2 O → 2 CH 3 COOHIf the rate law is assumed to be first order in acetic anhydride, the rate constant for this reaction can bedetermined by either measuring the decrease in the acetic anhydride concentration [R] or the increase inacetic acid concentration [P] with time since:−d[R]=dt12d[P]= k[R]dt(1)Because acetic anhydride has a strong absorbance at 228 nm and acetic acid does not, the acetic anhydrideconcentration can be conveniently determined by measuring the absorbance of the solution at thiswavelength. Unlike acetic anhydride, acetic acid is a weak electrolyte. Its concentration can bedetermined by measuring the conductance of the solution. If the same value for the rate constant isobtained using both methods, it confirms of the validity of the calculated value.Information about the reaction mechanism can be obtained through isotopic substitution. If H issubstituted with D and the ratio of the rate constants (k H /k D ) for the two reactions is >2, then this indicatesa primary kinetic isotope effect. This strongly suggests that the rate determining step in the reactionmechanism involves the breaking of a bond to the isotopically substituted atom. A secondary kineticisotope effect is of smaller magnitude (0.7 – 1.5) and can occur for a variety of other reasons. Forinstance, changing the solvent from water to D 2 O can affect the reaction rate if it changes the energy ofsolvation of either the reactants or the transition state complex. Unlike the primary isotope effect,secondary isotope effects can either cause an increase or a decrease in the reaction rate.In this experiment, the magnitude of the kinetic isotope effect is determined with UV-Vis spectroscopyusing a differential absorbance method. The reactions in D 2 O and H 2 O are carried out simultaneously inthe spectrometer: the water solution in a cuvette in the reference beam and the D 2 O solution in a cuvettein the sample beam. The difference in the absorption of the two solutions is monitored and the rateconstants for both reactions are determined through curve-fitting.1
(b) Determination of acetic acid concentrations from solution conductance measurements.The data collected in this experiment is the conductance (L) of the reaction mixture with time. Theconductance data must be converted into acetic acid concentrations in order to determine the rate constantusing equation (10).The conductivity κ of a solution is the product of the cell constant k and the solution conductance L. Itdepends upon the concentration and mobilities of the ions present in the solution:α cIκ = ( U + + U − )A B(11)1000where α is the fraction of the electrolyte which dissociates, c is the analytical concentration of theelectrolyte, I is Faraday’s constant, and U A+ and U B- are the true ionic mobilities of the ions present. Theconductance of a solution can also be described by the equivalent conductance Λ:1000κΛ = = α I(UcA+ +UB−)(12)For a strong electrolyte, α is independent of concentration, since α = 1 for all solute concentrations. Theion mobilities are concentration dependent, however: the higher the concentration, the more interactionbetween ions and the lower the ion mobilities. For a weak electrolyte, α varies with solute concentrationbut, since only a small portion of the solute ionizes, the mobilities are assumed to be largely independentof solute concentration. As a result, for a weak electrolyte:Λα ≅(13)where, Λ 0 is the equivalent conductance at infinite dilution. The equilibrium constant K for acetic acidcan be expressed in terms of equivalent conductances and the analytical concentration of acetic acid c HOAc :Λ 02c HOAcΛKHOAc=(14)Λ Λ − Λ)0 ( 0Using the relationship Λ = 1000kL/c HOAc and rearranging gives an expression which can be used todetermine the molar concentration of acetic acid from the conductance of the solution:1000kL 1000kL c HOAc = 1+(15)Λ0 K HOAc Λ0For acetic acid at 25 °C, the values for K HOAc and Λ 0 are 1.76 x 10 -5 and 390.55 cm 2 S mol -1 , respectively.The constant k for the conductance cell is 0.6 cm -1 . The conductances actually used in the equationshould be background corrected (L′) by subtracting the conductance measured when the reaction is begun(L 0 ) from each value (L′ = L – L 0 ).4
QuestionsReport the rate constants k H determined using the two methods. Do they agree? What does this suggestabout the assumption that the rate law is first order in acetic anhydride? Compare your results to aliterature value. How was the literature value determined? Discuss possible sources of error in theseexperiments.Sketch energy as a function of reaction coordinate for this reaction, showing the relative locations of thezero point energy levels for both the deuterated and protonated reactants, the transition state energy, andthe product energy. Also show the activation energy for the reaction and using your drawing, indicatewhether the reaction, as you’ve drawn it, is endothermic or exothermic.Assuming that the energy difference between the reactants and transition state complex is equal to theenergy required to break an O – H (or O – D) bond, estimate a value for the primary kinetic isotope effect(k H /k D ). HINTS: The difference in energy necessary to break these two bonds is equal to the difference intheir zero point vibrational energies. Force constants can be estimated from characteristic infraredabsorption frequencies. Also, remember that the Arrhenius equation relates activation energy to the rateconstant.Report the rate constants determined from the differential absorption data for the reaction in D 2 O (k D ) andthat for a water solution (k H ) and calculate the ratio: k H /k D . Is there evidence for a primary kinetic isotopeeffect? What does this say about the rate-determining step in the hydrolysis reaction? How does itcompare to the value estimated above? Propose a structure for the transition state complex for thisreaction.If your results suggest that the rate-determining step involves breaking a bond to H in H 2 O, why doesn’tthe rate law include the concentration of water?Include the graph of the conductance data and that of the differential absorption data with the best-fitcurve in your report. The graphs should be properly labeled and scaled and in a form suitable forpublication in a scientific paper.ReferencesDavis, K.R. and Hogg, J.L. J. Org. Chem. 1983, 48, 1041.Butler, A.R. and Gold, V. J. Chem. Soc. 1961, 2305.Batts, B.D. and Gold, V. J. Chem. Soc. A 1969, 984.Robertson, R.E., Rossall, B., and Redmond, W.A. Can. J. Chem. 1971, 49, 3665.Rossall, B. and Robertson, R.E. Can J. Chem. 1975, 53, 869.5