Download Now - The Burrill Report
Download Now - The Burrill Report
Download Now - The Burrill Report
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
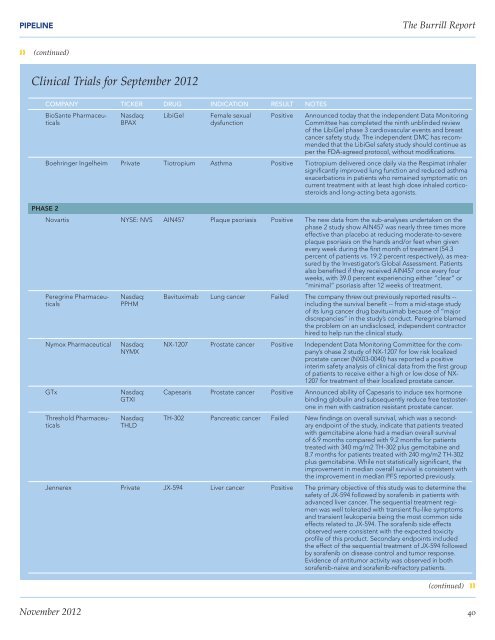
PIPELINE<strong>The</strong> <strong>Burrill</strong> <strong>Report</strong>❱❱ (continued)Clinical Trials for September 2012COMPANY TICKER DRUG INDICATION RESULT NOTESBioSante PharmaceuticalsPHASE 2Nasdaq:BPAXLibiGelFemale sexualdysfunctionPositiveAnnounced today that the independent Data MonitoringCommittee has completed the ninth unblinded reviewof the LibiGel phase 3 cardiovascular events and breastcancer safety study. <strong>The</strong> independent DMC has recommendedthat the LibiGel safety study should continue asper the FDA-agreed protocol, without modifications.Boehringer Ingelheim Private Tiotropium Asthma Positive Tiotropium delivered once daily via the Respimat inhalersignificantly improved lung function and reduced asthmaexacerbations in patients who remained symptomatic oncurrent treatment with at least high dose inhaled corticosteroidsand long-acting beta agonists.Novartis NYSE: NVS AIN457 Plaque psoriasis Positive <strong>The</strong> new data from the sub-analyses undertaken on thephase 2 study show AIN457 was nearly three times moreeffective than placebo at reducing moderate-to-severeplaque psoriasis on the hands and/or feet when givenevery week during the first month of treatment (54.3percent of patients vs. 19.2 percent respectively), as measuredby the Investigator’s Global Assessment. Patientsalso benefited if they received AIN457 once every fourweeks, with 39.0 percent experiencing either “clear” or“minimal” psoriasis after 12 weeks of treatment.Peregrine PharmaceuticalsNymox PharmaceuticalGTxThreshold PharmaceuticalsNasdaq:PPHMNasdaq:NYMXNasdaq:GTXINasdaq:THLDBavituximab Lung cancer Failed <strong>The</strong> company threw out previously reported results --including the survival benefit -- from a mid-stage studyof its lung cancer drug bavituximab because of “majordiscrepancies” in the study’s conduct. Peregrine blamedthe problem on an undisclosed, independent contractorhired to help run the clinical study.NX-1207 Prostate cancer Positive Independent Data Monitoring Committee for the company’sohase 2 study of NX-1207 for low risk localizedprostate cancer (NX03-0040) has reported a positiveinterim safety analysis of clinical data from the first groupof patients to receive either a high or low dose of NX-1207 for treatment of their localized prostate cancer.Capesaris Prostate cancer Positive Announced ability of Capesaris to induce sex hormonebinding globulin and subsequently reduce free testosteronein men with castration resistant prostate cancer.TH-302 Pancreatic cancer Failed New findings on overall survival, which was a secondaryendpoint of the study, indicate that patients treatedwith gemcitabine alone had a median overall survivalof 6.9 months compared with 9.2 months for patientstreated with 340 mg/m2 TH-302 plus gemcitabine and8.7 months for patients treated with 240 mg/m2 TH-302plus gemcitabine. While not statistically significant, theimprovement in median overall survival is consistent withthe improvement in median PFS reported previously.Jennerex Private JX-594 Liver cancer Positive <strong>The</strong> primary objective of this study was to determine thesafety of JX-594 followed by sorafenib in patients withadvanced liver cancer. <strong>The</strong> sequential treatment regimenwas well tolerated with transient flu-like symptomsand transient leukopenia being the most common sideeffects related to JX-594. <strong>The</strong> sorafenib side effectsobserved were consistent with the expected toxicityprofile of this product. Secondary endpoints includedthe effect of the sequential treatment of JX-594 followedby sorafenib on disease control and tumor response.Evidence of antitumor activity was observed in bothsorafenib-naive and sorafenib-refractory patients.(continued) ❱❱November 2012 40