perinatal-times_2013-03_print_and_read - SSM Cardinal Glennon ...
perinatal-times_2013-03_print_and_read - SSM Cardinal Glennon ...
perinatal-times_2013-03_print_and_read - SSM Cardinal Glennon ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
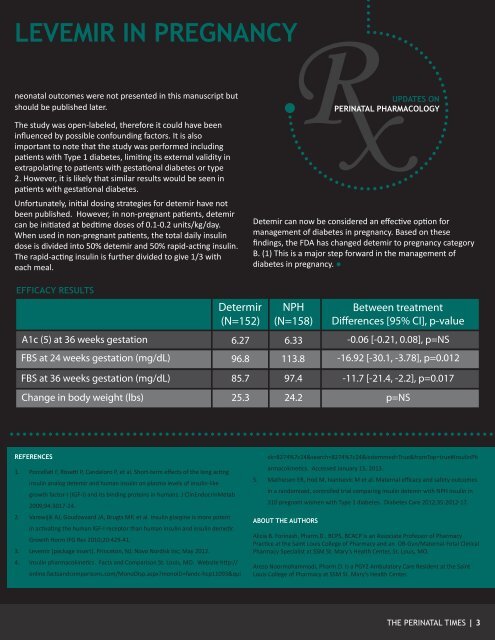
LEVEMIR IN PREGNANCYneonatal outcomes were not presented in this manuscript butshould be published later.The study was open-labeled, therefore it could have beeninfluenced by possible confounding factors. It is alsoimportant to note that the study was performed includingpatients with Type 1 diabetes, limiting its external validity inextrapolating to patients with gestational diabetes or type2. However, it is likely that similar results would be seen inpatients with gestational diabetes.Unfortunately, initial dosing strategies for detemir have notbeen published. However, in non-pregnant patients, detemircan be initiated at bedtime doses of 0.1-0.2 units/kg/day.When used in non-pregnant patients, the total daily insulindose is divided into 50% detemir <strong>and</strong> 50% rapid-acting insulin.The rapid-acting insulin is further divided to give 1/3 witheach meal.Detemir can now be considered an effective option formanagement of diabetes in pregnancy. Based on thesefindings, the FDA has changed detemir to pregnancy categoryB. (1) This is a major step forward in the management ofdiabetes in pregnancy. ●EFFICACY RESULTSREFERENCES1. Porcellati F, Rosetti P, C<strong>and</strong>eloro P, et al. Short-term effects of the long actinginsulin analog detemir <strong>and</strong> human insulin on plasma levels of insulin-likegrowth factor-I (IGF-I) <strong>and</strong> its binding proteins in humans. J ClinEndocrinMetab2009;94:3017-24.2. Varewijik AJ, Goudzwaard JA, Brugts MP, et al. Insulin glargine is more potentin activating the human IGF-I receptor than human insulin <strong>and</strong> insulin demetir.Growth Horm IFG Res 2010;20:429-41.3. Levemir [package insert]. Princeton, NJ: Novo Nordisk Inc; May 2012.4. Insulin pharmacokinetics. Facts <strong>and</strong> Comparison St. Louis, MO. Website http://online.facts<strong>and</strong>comparisons.com/MonoDisp.aspx?monoID=f<strong>and</strong>c-hcp11093&quick=8274%7c24&search=8274%7c24&isstemmed=True&fromTop=true#InsulinPharmacokinetics. Accessed January 15, <strong>2013</strong>.5. Mathiesen ER, Hod M, Ivanisevic M et al. Maternal efficacy <strong>and</strong> safety outcomesin a r<strong>and</strong>omized, controlled trial comparing insulin detemir with NPH insulin in310 pregnant women with Type 1 diabetes. Diabetes Care 2012;35:2012-17.ABOUT THE AUTHORSAlicia B. Forinash, Pharm.D., BCPS, BCACP is an Associate Professor of PharmacyPractice at the Saint Louis College of Pharmacy <strong>and</strong> an OB-Gyn/Maternal-Fetal ClinicalPharmacy Specialist at <strong>SSM</strong> St. Mary’s Health Center, St. Louis, MO.Arezo Noormohammadi, Pharm.D. is a PGY2 Ambulatory Care Resident at the SaintLouis College of Pharmacy at <strong>SSM</strong> St. Mary’s Health Center.THE PERINATAL TIMES | 3