Chapter 10 Modern Atomic Theory and the Periodic Table
Chapter 10 Modern Atomic Theory and the Periodic Table
Chapter 10 Modern Atomic Theory and the Periodic Table
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
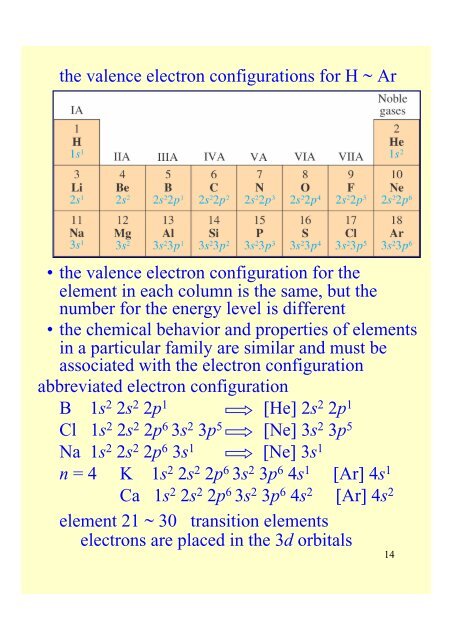
<strong>the</strong> valence electron configurations for H ~ Ar• <strong>the</strong> valence electron configuration for <strong>the</strong>element in each column is <strong>the</strong> same, but <strong>the</strong>number for <strong>the</strong> energy level is different• <strong>the</strong> chemical behavior <strong>and</strong> properties of elementsin a particular family are similar <strong>and</strong> must beassociated with <strong>the</strong> electron configurationabbreviated electron configurationB 1s 2 2s 2 2p 1 [He] 2s 2 2p 1Cl 1s 2 2s 2 2p 6 3s 2 3p 5 [Ne] 3s 2 3p 5Na 1s 2 2s 2 2p 6 3s 1 [Ne] 3s 1n = 4 K 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 [Ar] 4s 1Ca 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [Ar] 4s 2element 21 ~ 30 transition elementselectrons are placed in <strong>the</strong> 3d orbitals14










![Hetero [6+3] Cycloaddition of Fulvenes with N-Alkylidene Glycine ...](https://img.yumpu.com/35423358/1/190x245/hetero-6-3-cycloaddition-of-fulvenes-with-n-alkylidene-glycine-.jpg?quality=85)




