Chapter 10 Modern Atomic Theory and the Periodic Table
Chapter 10 Modern Atomic Theory and the Periodic Table
Chapter 10 Modern Atomic Theory and the Periodic Table
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
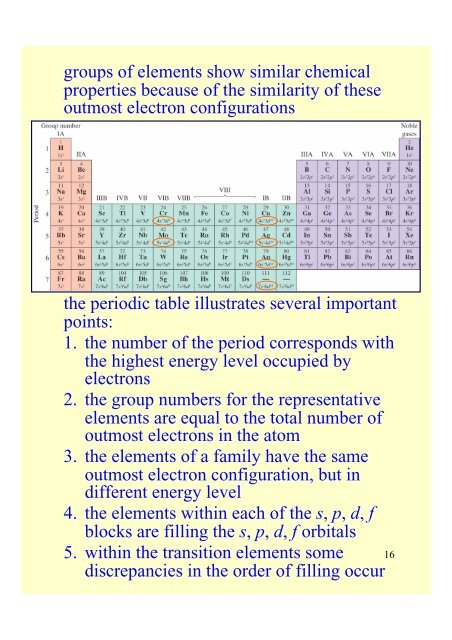
groups of elements show similar chemicalproperties because of <strong>the</strong> similarity of <strong>the</strong>seoutmost electron configurations<strong>the</strong> periodic table illustrates several importantpoints:1. <strong>the</strong> number of <strong>the</strong> period corresponds with<strong>the</strong> highest energy level occupied byelectrons2. <strong>the</strong> group numbers for <strong>the</strong> representativeelements are equal to <strong>the</strong> total number ofoutmost electrons in <strong>the</strong> atom3. <strong>the</strong> elements of a family have <strong>the</strong> sameoutmost electron configuration, but indifferent energy level4. <strong>the</strong> elements within each of <strong>the</strong> s, p, d, fblocks are filling <strong>the</strong> s, p, d, f orbitals5. within <strong>the</strong> transition elements some 16discrepancies in <strong>the</strong> order of filling occur










![Hetero [6+3] Cycloaddition of Fulvenes with N-Alkylidene Glycine ...](https://img.yumpu.com/35423358/1/190x245/hetero-6-3-cycloaddition-of-fulvenes-with-n-alkylidene-glycine-.jpg?quality=85)




