ECCnet Registry Release Notes 10.5 - GS1 Canada
ECCnet Registry Release Notes 10.5 - GS1 Canada
ECCnet Registry Release Notes 10.5 - GS1 Canada
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
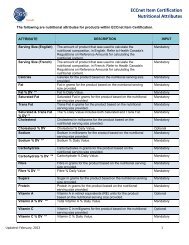
6 DATA REMEDIATION6.1 Data Remediation for Suppliers That Have Market GroupsAs a part of <strong>ECCnet</strong> <strong>Registry</strong> <strong>Release</strong> <strong>10.5</strong>, <strong>GS1</strong> <strong>Canada</strong> will remediate on behalf of all Suppliersthat have a Third Party as a Data Recipient in any of the Market Groups.These Data Recipients will be removed from the Market Groups, as a part of the data remediationprocess. Third Party Data Recipients will remain as the Supplier’s trading partners.Important Note: Suppliers that object to data remediation to be performed on their behalf by <strong>GS1</strong><strong>Canada</strong> must notify and request <strong>GS1</strong> <strong>Canada</strong> to keep their Third Party Data Recipients in theirmarket groups. The request should be sent by email to customer support at<strong>ECCnet</strong>Support@gs1ca.org no later than September 28, 2012.The email subject line should include the words “Third Party Data Recipient”.6.2 Data Remediation for Retired Code List ValuesIf one or more of the retired code list values are currently available in a Supplier’s product data,the product’s compliancy status will be set to ‘non-compliant’, which will result in datasynchronization being stopped. Suppliers will need to provide new values for those attributes.For example, if Dosage Form attribute value is currently set to ‘Aerosol, solution’, the status of theproduct will be ‘non-compliant, because ‘Aerosol, solution’ will no longer be a valid value forDosage Form attribute. The Supplier will have to select a new value from the list of Valid CodeList Values for Dosage Form attribute to attain ‘compliant’ status for the product. Please see the<strong>ECCnet</strong> <strong>Registry</strong> Attributes Guide – Upcoming <strong>ECCnet</strong> <strong>Registry</strong> <strong>10.5</strong> <strong>Release</strong> for the Valid CodesList Values in <strong>ECCnet</strong> <strong>Registry</strong>.7 APPENDIX A: ECCNET REGISTRY ATTRIBUTES (FOR RELEASE<strong>10.5</strong>)Please refer to the link below for a list of <strong>ECCnet</strong> <strong>Registry</strong> Attributes and Code Lists for theupcoming <strong>Release</strong> <strong>10.5</strong>. <strong>ECCnet</strong> <strong>Registry</strong> Attributes Guide – Upcoming <strong>ECCnet</strong> <strong>Registry</strong> <strong>10.5</strong><strong>Release</strong>.8 APPENDIX B: CODE LIST VALUES UPDATESPlease see all <strong>ECCnet</strong> Valid Codes List Values in the <strong>ECCnet</strong> <strong>Registry</strong> Attributes Guide –Upcoming <strong>ECCnet</strong> <strong>Registry</strong> <strong>10.5</strong> <strong>Release</strong>.8.1 New Packaging Material Code List Values<strong>ECCnet</strong> <strong>Registry</strong> <strong>Release</strong> <strong>Notes</strong> <strong>10.5</strong> Page 10 of 21