ECCnet Registry Release Notes 10.5 - GS1 Canada
ECCnet Registry Release Notes 10.5 - GS1 Canada
ECCnet Registry Release Notes 10.5 - GS1 Canada
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
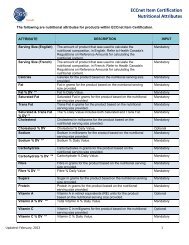
Template) will be supported until the next major <strong>ECCnet</strong> <strong>Release</strong> (<strong>ECCnet</strong> <strong>Release</strong>10.6).Please note that there will no longer be mutually exclusive validation for DIN and NPN.This is because the new structure of Drug and Health Product Details section willprevent entering both DIN and NPN for the same Drug and Health Product Detailssection. As a result, if Suppliers, using the Pre-<strong>Release</strong> <strong>10.5</strong> DIY Rx2 template, willprovide both DIN and NPN for the same Drug and Health Product Details section, onlyNPN will be accepted and saved in <strong>ECCnet</strong> <strong>Registry</strong>. DIN will be dropped, and no errormessage will be returned.10 APPENDIX D: DATA IMPORT TOOL (DIT) SUPPLIER IMPACTSUMMARY• Each DIT Supplier user will have to accept the End User License Agreement (EULA) atthe time of first log in to <strong>ECCnet</strong> ProSYNC®.• DIT Suppliers will be able to provide new values for attributes:o Packaging MaterialooSpecial Handling – StorageSpecial Handling – Transportation(See Appendix B: Code List Values Updates)• Retired attributes, if provided via DIT, will be dropped (see section 4 – Retired Attributesfor details).• Retired code list values will be considered ‘invalid’, if provided via DIT (see Appendix B:Code List Values Updates), and the GTIN will be ‘non-compliant’.• DIT Suppliers will be able to provide values for Flashpoint Temperature within the rangeof -999.9999 to 9999.9999• DIT Suppliers will be able to provide multiple AHFS codes by entering multiple commaseparatedcodes into AHFS field in the Advanced_Rx2 Template.• DIT Suppliers will be able to provide multiple DIN-HM, DIN and NPN values in a newstructure that employs new attributes Drug and Health Product Number Type andDrug and Health Product Number (see New Attributes and Changed attributesections of this document for more details).New attributes will be available in Advanced_Rx2 template. A Supplier will enter eachDrug and Health Product Number with a corresponding Drug and Health ProductNumber Type in a separate row.Please note that a new version of Data Import Tool (version <strong>10.5</strong>) will be available forDIT users. The new version will be supporting the new structure for DIN, NPN and DIN-HM that involves the introduction of new attributes, Drug and Health Product Number,Drug and Health Product Number Type and multiple AHFS codes (see both the NewAttributes and Changed attributes sections of this document for more details). The oldData Import Tool version 9.6 will still be supported until a future <strong>ECCnet</strong> <strong>Registry</strong><strong>Release</strong>.<strong>ECCnet</strong> <strong>Registry</strong> <strong>Release</strong> <strong>Notes</strong> <strong>10.5</strong> Page 18 of 21