cubic close-packed structure - IUT Annecy
cubic close-packed structure - IUT Annecy
cubic close-packed structure - IUT Annecy
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
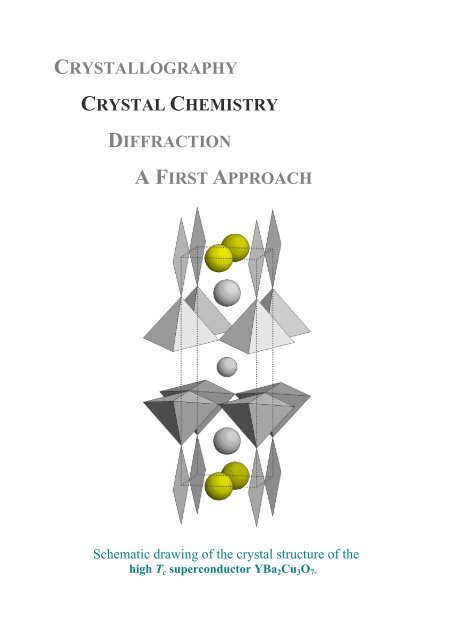
CRYSTALLOGRAPHYCRYSTAL CHEMISTRYDIFFRACTIONA FIRST APPROACHSchematic drawing of the crystal <strong>structure</strong> of thehigh T c superconductor YBa 2 Cu 3 O 7-
Type ofcompoundIoniccompoundsCompoundswith anextendedlattice ofcovalentbondsMolecularcompoundsMetalliccompoundsCLASSIFICATION OF CRYSTALStructuralunitsCations &anionsAtomsMoleculesAtomsSTRUCTURESChemical bondsElectricalattraction,Non localisedMainly covalentbondsMainly covalentbonds insidemolecules, van derWaals interactionsor hydrogen bondsbetween moleculesBand <strong>structure</strong>,Non localisedbondsCharacteristicpropertiesHard & brittle,High meltingpoints, ModerateinsulatorsHard, Highmelting points,InsulatorsModeratemelting point,High thermalexpansioncoefficient,InsulatorsDuctility, Highmelting point ingeneral, GoodelectricalconductivityExamplesSalts (NaCl,CsCl)Diamond(C), Quartz(SiO 2 ),Cellulose(C 6 H 10 O 5 )Ice (H 2 O),Naphthalene(C 10 H 8 ),Aspirin(C 9 H 8 O 4 )Iron,Aluminium,Copper,SodiumSeveral types of atomic radii are defined depending on the nature ofbonds within the crystal:- The covalent radius corresponds to half of the distance betweentwo identical atomic nuclei, bound by a covalent bond. It isdetermined using A 2 vapours or crystal <strong>structure</strong>s like diamond,silicon, germanium …- The ionic radius is estimated from the distance between cationsand anions that are adjacent in ion crystals. It is assumed that theinter-nuclear distance between two ions in the ionic <strong>structure</strong> isequal to the sum of the radius of those ions, though the ionic radiusStructure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 38
is not actually constant because it depends on the ionic valence, andthe coordination number.- The metallic radius is one-half of the <strong>close</strong>st internuclear distancein a metallic crystal.- Van der Waals radii are determined from measurements of atomicspacing between pairs of unbonded atoms in crystals.ExamplesCl [Å] Cu [Å]Ionic radii 1.67 (-1) 0.77 (+1) VI0.73 (+2) VI0.54 (+3) VIcovalent radii 1.24 1.22metallic radius 1.28van der Waals radius 1.75Inter-atomic distancesd = [(a∆x)² + (b∆y)² + (c∆z)² + 2bc∆y∆zcosα + 2ca∆z∆xcosβ + 2ab∆x∆ycosγ] 1/2with ∆x = x 2 – x 1 , ∆y = y 2 – y 1 , ∆z = z 2 - z 1Cubic system: d = a [(∆x)² + (∆y)² + (∆z)²] 1/2Bond angles:cos ϕ = (d² 12 + d² 23 – d² 13 ) / 2d 12 d 23Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 39
CLOSE PACKINGWe consider here that atoms are non-penetrating hard spheres.Hexagonal layer of atomsEach sphere has six neighbours that form a perfect hexagon and is incontact with them.Stacking sequenceClose-<strong>packed</strong> <strong>structure</strong>s are obtained by stacking such layers so thatthe atoms of the upper layer are located in the “hollows” or intersticebetween the atoms of the lower layer. For example, if the first layerhas atoms in positions A (A layer), the atoms of the second layer maybe placed in the interstices marked B (B layer).When a third layer is placed upon, there are two possibilities:- the third layer goes directly above the A layer. The stackingsequence is ABABABA… (hexagonal <strong>close</strong>-<strong>packed</strong> <strong>structure</strong>),- the third layer of atoms goes above the interstice marked C (Clayer). The stacking sequence is now ABCABCABC… (<strong>cubic</strong><strong>close</strong>-<strong>packed</strong> <strong>structure</strong>).Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 40
Hexagonal <strong>close</strong> packing (hcp)ABABABABABAB…..Unit cell:Hexagonal lattice P2 atoms: 0 0 0 ; 2/3 1/3 1/2a = 2r ; c = 4 (2/3) 1/2 rc/a = 2 √2 / √3 = 1.63Number of nearest neighbours : 6 + 3 + 3 = 12 (anti-cuboctahedron) ata distance 2rStructure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 41
Cubic <strong>close</strong> packing (ccp)ABCABCABCABC…Unit cell:Cubic lattice F4 atoms: 0 0 0 ; (1/2 1/2 0 ; 1/2 0 1/2 ; 0 1/2 1/2)a = 2√2 rNumber of nearest neighbours : 6 + 3 + 3 = 12 (cuboctahedron) at 2rStructure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 42
Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 43
Dense <strong>structure</strong>sAtomic Packing factor (packing fraction, packing efficiency) : 74 %NAPF =atomsV× Vunit cellNumber of nearest neighbours : 12 at 2rHexagonal <strong>close</strong> packing : Mg, Ti, Co …Cubic <strong>close</strong> packing : Cu, Ca, Al …Some larger structural units form crystal <strong>structure</strong>s based on theprinciple of <strong>close</strong> packing.atomsBuckminsterfullerene, C 60Cubic <strong>close</strong> packing (fcc) of C 60 spheres(12 pentagons & 20 hexagons = truncated icosahedron)Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 44
Interstices in <strong>close</strong>-<strong>packed</strong> <strong>structure</strong>sr = radius of the atom forming the <strong>close</strong>-<strong>packed</strong> <strong>structure</strong>Octahedral interstice (octahedral interstitial site) : r oct. = 0.414 rIn a <strong>close</strong>-<strong>packed</strong> <strong>structure</strong>, the number of octahedral interstitial sitesis equal to the number of atoms.Cubic <strong>close</strong>-<strong>packed</strong> (Cubic F) Hexgonal <strong>close</strong>-<strong>packed</strong>(Hexagonal P)Atoms (blue): 0 0 0 (+ F) 0 0 0 ; 2/3 1/3 1/2Sites (yellow): 1/2 0 0 (+ F) 1/3 2/3 1/4 ; 1/3 2/3 3/4Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 45
Tetrahedral interstice (or tetrahedral interstitial site): r tetr. = 0.225 rIn a <strong>close</strong>-<strong>packed</strong> <strong>structure</strong>, the number of tetrahedral interstitial sitesis twice the number of atoms.Cubic <strong>close</strong>-<strong>packed</strong> (Cubic F)Atoms (blue): 0 0 0 (+ F)Sites (yellow): 1/4 1/4 1/4 (+ F)1/4 1/4 3/4 (+ F)Hexagonal <strong>close</strong>-<strong>packed</strong> (Hexagonal P)Atoms (blue): 0 0 0 ; 2/3 1/3 1/2Sites (yellow): {0 0 3/8 ; 2/3 1/3 7/8} ; {0 0 5/8 ; 2/3 1/3 1/8}Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 46
Dense <strong>structure</strong>s with occupied interstitial sites (interstitial <strong>structure</strong>s)Interstitial compounds: metallic array + H, C, N …Iono-covalent compounds : anionic array + cationsCompound Close packing Number of occupied Number of occupiedoctahedral interstices tetrahedral intersticesNaCl Cubic (Cl) 1 / 1 -ZnS wurtzite Hexagonal (S) - 1 / 2Fe 4 N Hexgonal (Fe) 1 / 4 -Fe 4 C Cubic (Fe) - 1 / 8Body centred <strong>cubic</strong> packingBody centred <strong>cubic</strong> lattice : APF 68 %Each sphere is in contact with 4 spheres in the upper layer and 4spheres in the lower layer. It is however not in contact withneighbouring spheres in its own layer. This is the reason why the APFis only 68 % and not 74 % as in <strong>close</strong>-<strong>packed</strong> <strong>structure</strong>.Unit cell:Cubic lattice I2 atoms: 0 0 0 ; (1/2 1/2 1/2)a = 4r/√3Number of nearest neighbours : 4 + 4 = 8 (cube) at 2rSome larger structural units form crystal <strong>structure</strong>s based on theprinciple of body centred <strong>cubic</strong> packing.Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 47
ATOM COORDINATIONCoordiantion number: number of neighbouring atomsCoordination polyhedron: polyhedron formed by these atomsMain coordination polyhedra observed in ionic or covalent solids aswell as in molecules.GdMo 16 O 44R 3, a = 10.780(2) Å, c = 27.493(4) Å, Z = 3, D x = 4.31 Mg m -3Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 48
THE ION COMPOUNDSThe anions are generally larger than the cations with similar atomicnumber. The small cations “fit” into the interstices between largeanions.The <strong>structure</strong> mainly depends on the larger species which createsinterstitial sites.It also depends on the ratio between the species radii (cation radius /anion radius – geometrical criterion) which gives the cationcoordination.Pauling’s first ruleA coordination polyhedron of anions is formed around every cation(and vice-versa) - it will only be stable if the cation is in contact witheach of its neighbours. Ionic crystals may thus be considered as sets of linked polyhedra. The cation-anion distance is regarded as the sum of the ionic radii.Radii of interstices between anions:r tetrahedral site = 0.225 r anionr octahedral site = 0.414 r anionr <strong>cubic</strong> site = 0.732 r anionThese values correspond to the case when anions are tangent spheres(<strong>close</strong> packing).One cannot bring the anions <strong>close</strong>r to each other.The radius of a cation occupying an interstice must be equal toor larger than the radius of the interstice. The ratio r interstice / r anion is a minimum value for the ratio r cation /r anion below which the interstice will not be occupied and thecorresponding coordination not realised.Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 49
In other words, the interstitial site is occupied only if:rrrrcationanionr≥rintersticeintersticewith: = 0.225 (Tetra. site), 0.414 (Octa. site), 0.732 (Cubic site)anionCoordiantion Minimum ratio forExamplesr cation /r anion Compound r cation /r anionTetrahedral 0.225 ZnS Blende 0.25Octahedral 0.414 NaCl 0.52Cubic 0.732 CsCl 0.93anionZnS (Zinc blende) NaCl CsClThe energy associated with an ionic bond, i.e. the energy required toseparate completely a mole of a solid ionic compound into its gaseousions, is several hundreds of kJ mol -1 .Salts with Cubic F Bravais LatticeStructure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 50
Covalent bondsTHE COVALENT COMPOUNDSIn covalent compounds with iono-covalent bonds (several hundreds ofkJ.mol -1 ), the coordination is given by the electronic configuration.Coordiantion Electronic ExampleconfigurationLinear (2) sp, d 10 HgCl 2 , Cu 2 OTrigonal planar (3) sp 2 CaCO 3Square planar (4) d 8 PdCl 2Tetrahedral (4) sp 3 CF 4Examples of SiO 4 tetrahedra arrangements observed in silicates.Double chains [Si 2 O 5 ] Ring [Si 18 O 54 ]Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 51
The hydrogen bondThe hydrogen bond is a special case of dipole forces. A hydrogenbond is the attractive force between the hydrogen attached to anelectronegative atom of one molecule and an electronegative atom of adifferent molecule. Usually the electronegative atom is oxygen,nitrogen, or fluorine, which has a partial negative charge. Thehydrogen then has the partial positive charge. ( ⎯⎯ H -------).The hydrogen bond holds polarised organic molecules together. Theenergy associated with the hydrogen bond is several tens of kJ mol -1 .Van der Waals’ forcesVan der Waals' forces (sometimes called London dispersion forces) isa class of intermolecular forces which arise from the polarisation ofmolecules into dipôles. This includes forces that arise from fixed orangle-averaged dipoles (Keesom forces) and free or rotation dipoles(Debye forces) as well as shifts in electron cloud distribution (Londonforces). The energy implied is several kJ.mol -1 .Van der Waals' forces are observed in noble gases.Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 52
Structure of non-metallic elementsArgon, Ar (Ne, Kr, Xe)Cubic, Fm3ma = 5.31 Å, Z = 4Ar 0 0 0Number of bonds : 0Iodine, I 2 (Cl 2 , Br 2 )Orthorhombic, Cmcaa = 7.14, b = 4.69, c = 9.78 Å,Z = 4I 0 0.154 0.117Number of bonds : 1Selenium, Se (Te)Trigonal, P3 1 21a = 4.37, c = 4.95 Å, Z = 3Se 0.225 0 1/3Number of bonds : 2Arsenic, As (P, Sb, Bi)Trigonal, R3ma = 3.76, c = 10.55 Å, Z = 6As 0 0 0.227Number of bonds : 3Diamond, C (Si, Ge, Sn)Cubic, Fm3ma = 3.57 Å, Z = 8C 1/8 1/8 1/8Number of bonds : 4Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 53
THE METALLIC COMPOUNDSNearly all metals crystallise with hexagonal <strong>close</strong> packing, <strong>cubic</strong> <strong>close</strong>packing or body-centred <strong>cubic</strong> packing.- hcp: Mg, Sc, Zr , TI, Co, Nd …- ccp: Al, Ni, Ag, Cu, Au, Pt …- bcc : Ba, Fe, Cr, V, Mo, W, Alkali metals …Periodic Table with <strong>structure</strong> of elements12 12 12 16anti-cuboctahedron cuboctahedron icosahedronhcpccpStructure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 54
Structure type:STRUCTURE TYPESSpecific geometrical arrangement of atoms.Two <strong>structure</strong>s are considered isotypic if they have the samestoichiometry, the same space group, the same Wyckoff sites with thesame or similar positional coordinates and the same or similar valuesof the unit cell axial ratios and cell angles.A <strong>structure</strong> type is generally denoted after the first identifiedcompound that has the corresponding specific geometricalarrangement of atoms.Cu W MgA1 A2 A3NaCl CsCl ZnS (Zinc blende)B1 B2 B3There are about 8000 different <strong>structure</strong> types for inorganiccompounds. Several hundreds are discovered each year.Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 55
SUBSTANCES WITH DIFFERENTCRYSTAL STRUCTURESSome substances may have different <strong>structure</strong>s depending on thetemperature and the applied pressure.Influence of temperatureAn increase in temperature generally results in:- an increased unit cell volume (positive expansion coefficient),- a higher symmetry,- a partial disorder.Influence of applied pressureAn increase in pressure results:- in a reduced unit cell volume (neg. compressibility coefficient),- in an increased Atomic Packing Factor,- often in higher coordination number.Iron phase diagram: the Greek letters denote different <strong>structure</strong>s.Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 56
Polytypic substance:An element or compound is polytypic if it occurs in several structuralmodifications, each of which can be regarded as built up by stackinglayers of (nearly) identical <strong>structure</strong> and composition, and if themodifications differ only in their stacking sequence. Polytypism is aspecial case of polymorphism: the two-dimensional translations withinthe layers are essentially preserved (e.g. SiC, ZnS).Allotropic modifications : Different crystal <strong>structure</strong>s for a givenchemical element.DiamondGraphiteSolid solutionA homogeneous crystalline <strong>structure</strong> in which one or more types ofatoms or molecules may be partly and randomly substituted for theoriginal atoms and molecules without changing the <strong>structure</strong> (Ni-Cu).Partial disorderOrdered alloyDisordered alloyStructure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 57
STRUCTURE RELATIONSSome crystal <strong>structure</strong>s are related to others that are simpler by suchoperations as deformation, substitution, insertion of additionalatoms or elimination of atoms.Others may result from different stacking sequences of commonstructural units. The arrangement of several structural units in varyingproportions leads to <strong>structure</strong> series.Deformation derivativesAs temperature is decreased, BaTiO 3 undergoes several <strong>structure</strong>transformations due to the displacement of cations with respect to theanionic matrix. The <strong>cubic</strong> <strong>structure</strong> with perovskite type becomestetragonal, orthorhombic and finally trigonal.Cubic P Tetragonal P Orthorhombic C Trigonal RT (K) ——— 393 ————— 278 ——————— 183 ————Substitution derivativesW, Cubic I CsCl, Cubic Pa CsCl = a WMnCu 2 Al, Cubic Fa MnCu2Al = 2 a WStructure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 58
Vacancy derivatives & derivatives with occupied interstitial sitesStructure seriesK 3 C 60Fm3mn(CuO 2 ) = 1TlBa 2 CuO 5n(CuO 2 ) = 2TlBa 2 CaCu 2 O 7n(CuO 2 ) = 3TlBa 2 Ca 2 Cu 3 O 9n(CuO 2 ) = 4TlBa 2 Ca 3 Cu 4 O 11High T c superconductor series TlBa 2 Ca n-1 Cu n O 2n+3Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 59
Intergrowth <strong>structure</strong>sYBa 2 Cu 3 O 7YBa 2 Cu 4 O 8YBa 2 Cu 3 O 7 ⊕ YBa 2 Cu 4 O 8 ≡ Y 2 Ba 4 Cu 7 O 15Structure of Materials – <strong>IUT</strong> <strong>Annecy</strong> – Mesures Physiques – Part II 60