Physics
Physics
Physics
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
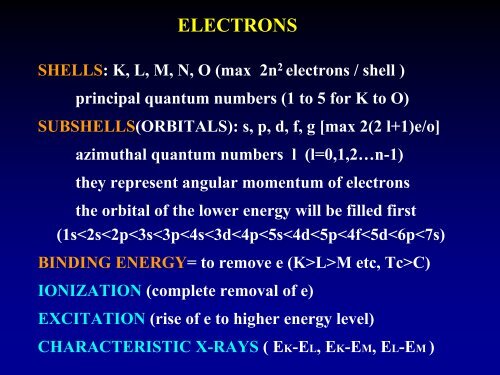
ELECTRONSSHELLS: K, L, M, N, O (max 2n 2 electrons / shell )principal quantum numbers (1 to 5 for K to O)SUBSHELLS(ORBITALS): s, p, d, f, g [max 2(2 l+1)e/o]azimuthal quantum numbers l (l=0,1,2…n-1)they represent angular momentum of electronsthe orbital of the lower energy will be filled first(1s