What is the theoretical background of potentiometry? - Metrohm
What is the theoretical background of potentiometry? - Metrohm
What is the theoretical background of potentiometry? - Metrohm
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
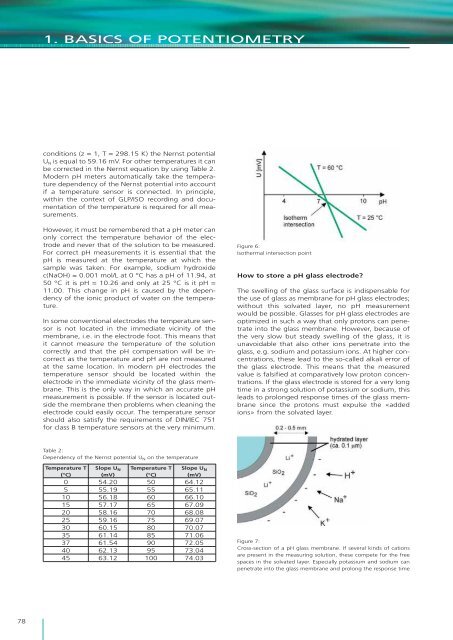
1. BASICS OF POTENTIOMETRYconditions (z = 1, T = 298.15 K) <strong>the</strong> Nernst potentialU N <strong>is</strong> equal to 59.16 mV. For o<strong>the</strong>r temperatures it canbe corrected in <strong>the</strong> Nernst equation by using Table 2.Modern pH meters automatically take <strong>the</strong> temperaturedependency <strong>of</strong> <strong>the</strong> Nernst potential into accountif a temperature sensor <strong>is</strong> connected. In principle,within <strong>the</strong> context <strong>of</strong> GLP/ISO recording and documentation<strong>of</strong> <strong>the</strong> temperature <strong>is</strong> required for all measurements.However, it must be remembered that a pH meter canonly correct <strong>the</strong> temperature behavior <strong>of</strong> <strong>the</strong> electrodeand never that <strong>of</strong> <strong>the</strong> solution to be measured.For correct pH measurements it <strong>is</strong> essential that <strong>the</strong>pH <strong>is</strong> measured at <strong>the</strong> temperature at which <strong>the</strong>sample was taken. For example, sodium hydroxidec(NaOH) = 0.001 mol/L at 0 °C has a pH <strong>of</strong> 11.94, at50 °C it <strong>is</strong> pH = 10.26 and only at 25 °C <strong>is</strong> it pH =11.00. Th<strong>is</strong> change in pH <strong>is</strong> caused by <strong>the</strong> dependency<strong>of</strong> <strong>the</strong> ionic product <strong>of</strong> water on <strong>the</strong> temperature.In some conventional electrodes <strong>the</strong> temperature sensor<strong>is</strong> not located in <strong>the</strong> immediate vicinity <strong>of</strong> <strong>the</strong>membrane, i.e. in <strong>the</strong> electrode foot. Th<strong>is</strong> means thatit cannot measure <strong>the</strong> temperature <strong>of</strong> <strong>the</strong> solutioncorrectly and that <strong>the</strong> pH compensation will be incorrectas <strong>the</strong> temperature and pH are not measuredat <strong>the</strong> same location. In modern pH electrodes <strong>the</strong>temperature sensor should be located within <strong>the</strong>electrode in <strong>the</strong> immediate vicinity <strong>of</strong> <strong>the</strong> glass membrane.Th<strong>is</strong> <strong>is</strong> <strong>the</strong> only way in which an accurate pHmeasurement <strong>is</strong> possible. If <strong>the</strong> sensor <strong>is</strong> located outside<strong>the</strong> membrane <strong>the</strong>n problems when cleaning <strong>the</strong>electrode could easily occur. The temperature sensorshould also sat<strong>is</strong>fy <strong>the</strong> requirements <strong>of</strong> DIN/IEC 751for class B temperature sensors at <strong>the</strong> very minimum.Figure 6:Iso<strong>the</strong>rmal intersection pointHow to store a pH glass electrode?The swelling <strong>of</strong> <strong>the</strong> glass surface <strong>is</strong> ind<strong>is</strong>pensable for<strong>the</strong> use <strong>of</strong> glass as membrane for pH glass electrodes;without th<strong>is</strong> solvated layer, no pH measurementwould be possible. Glasses for pH glass electrodes areoptimized in such a way that only protons can penetrateinto <strong>the</strong> glass membrane. However, because <strong>of</strong><strong>the</strong> very slow but steady swelling <strong>of</strong> <strong>the</strong> glass, it <strong>is</strong>unavoidable that also o<strong>the</strong>r ions penetrate into <strong>the</strong>glass, e.g. sodium and potassium ions. At higher concentrations,<strong>the</strong>se lead to <strong>the</strong> so-called alkali error <strong>of</strong><strong>the</strong> glass electrode. Th<strong>is</strong> means that <strong>the</strong> measuredvalue <strong>is</strong> falsified at comparatively low proton concentrations.If <strong>the</strong> glass electrode <strong>is</strong> stored for a very longtime in a strong solution <strong>of</strong> potassium or sodium, th<strong>is</strong>leads to prolonged response times <strong>of</strong> <strong>the</strong> glass membranesince <strong>the</strong> protons must expulse <strong>the</strong> «addedions» from <strong>the</strong> solvated layer.Table 2:Dependency <strong>of</strong> <strong>the</strong> Nernst potential U N on <strong>the</strong> temperatureTemperature T Slope U N Temperature T Slope U N(°C) (mV) (°C) (mV)0 54.20 50 64.125 55.19 55 65.1110 56.18 60 66.1015 57.17 65 67.0920 58.16 70 68.0825 59.16 75 69.0730 60.15 80 70.0735 61.14 85 71.0637 61.54 90 72.0540 62.13 95 73.0445 63.12 100 74.03Figure 7:Cross-section <strong>of</strong> a pH glass membrane. If several kinds <strong>of</strong> cationsare present in <strong>the</strong> measuring solution, <strong>the</strong>se compete for <strong>the</strong> freespaces in <strong>the</strong> solvated layer. Especially potassium and sodium canpenetrate into <strong>the</strong> glass membrane and prolong <strong>the</strong> response time78