What is the theoretical background of potentiometry? - Metrohm
What is the theoretical background of potentiometry? - Metrohm
What is the theoretical background of potentiometry? - Metrohm
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
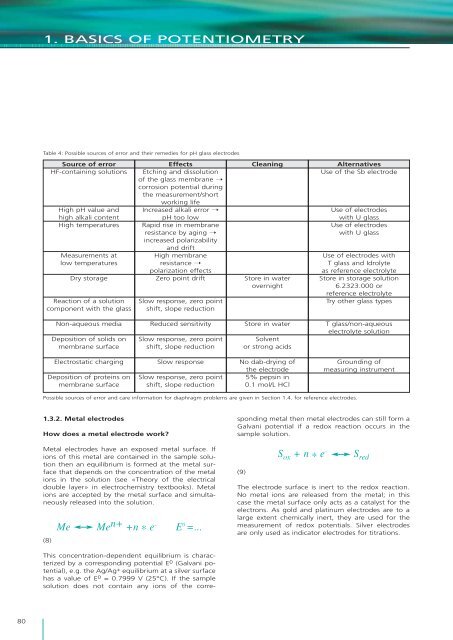
1. BASICS OF POTENTIOMETRYTable 4: Possible sources <strong>of</strong> error and <strong>the</strong>ir remedies for pH glass electrodesSource <strong>of</strong> errorHF-containing solutionsHigh pH value andhigh alkali contentHigh temperaturesMeasurements atlow temperaturesDry storageReaction <strong>of</strong> a solutioncomponent with <strong>the</strong> glassEffectsEtching and d<strong>is</strong>solution<strong>of</strong> <strong>the</strong> glass membrane ➝corrosion potential during<strong>the</strong> measurement/shortworking lifeIncreased alkali error ➝pH too lowRapid r<strong>is</strong>e in membraneres<strong>is</strong>tance by aging ➝increased polarizabilityand driftHigh membraneres<strong>is</strong>tance ➝polarization effectsZero point driftSlow response, zero pointshift, slope reductionCleaningStore in waterovernightAlternativesUse <strong>of</strong> <strong>the</strong> Sb electrodeUse <strong>of</strong> electrodeswith U glassUse <strong>of</strong> electrodeswith U glassUse <strong>of</strong> electrodes withT glass and Idrolyteas reference electrolyteStore in storage solution6.2323.000 orreference electrolyteTry o<strong>the</strong>r glass typesNon-aqueous mediaDeposition <strong>of</strong> solids onmembrane surfaceReduced sensitivitySlow response, zero pointshift, slope reductionStore in waterSolventor strong acidsT glass/non-aqueouselectrolyte solutionElectrostatic chargingDeposition <strong>of</strong> proteins onmembrane surfaceSlow responseSlow response, zero pointshift, slope reductionNo dab-drying <strong>of</strong><strong>the</strong> electrode5% pepsin in0.1 mol/L HClGrounding <strong>of</strong>measuring instrumentPossible sources <strong>of</strong> error and care information for diaphragm problems are given in Section 1.4. for reference electrodes.1.3.2. Metal electrodesHow does a metal electrode work?Metal electrodes have an exposed metal surface. Ifions <strong>of</strong> th<strong>is</strong> metal are contained in <strong>the</strong> sample solution<strong>the</strong>n an equilibrium <strong>is</strong> formed at <strong>the</strong> metal surfacethat depends on <strong>the</strong> concentration <strong>of</strong> <strong>the</strong> metalions in <strong>the</strong> solution (see «Theory <strong>of</strong> <strong>the</strong> electricaldouble layer» in electrochem<strong>is</strong>try textbooks). Metalions are accepted by <strong>the</strong> metal surface and simultaneouslyreleased into <strong>the</strong> solution.(8)Me Me n+ +n * e – E 0 =...Th<strong>is</strong> concentration-dependent equilibrium <strong>is</strong> characterizedby a corresponding potential E 0 (Galvani potential),e.g. <strong>the</strong> Ag/Ag + equilibrium at a silver surfacehas a value <strong>of</strong> E 0 = 0.7999 V (25°C). If <strong>the</strong> samplesolution does not contain any ions <strong>of</strong> <strong>the</strong> correspondingmetal <strong>the</strong>n metal electrodes can still form aGalvani potential if a redox reaction occurs in <strong>the</strong>sample solution.(9)S ox + n * e –S redThe electrode surface <strong>is</strong> inert to <strong>the</strong> redox reaction.No metal ions are released from <strong>the</strong> metal; in th<strong>is</strong>case <strong>the</strong> metal surface only acts as a catalyst for <strong>the</strong>electrons. As gold and platinum electrodes are to alarge extent chemically inert, <strong>the</strong>y are used for <strong>the</strong>measurement <strong>of</strong> redox potentials. Silver electrodesare only used as indicator electrodes for titrations.80