Cerebral Oximetry Monitoring Clinical Protocol - Casecag.com
Cerebral Oximetry Monitoring Clinical Protocol - Casecag.com
Cerebral Oximetry Monitoring Clinical Protocol - Casecag.com
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
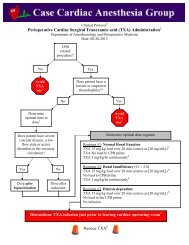
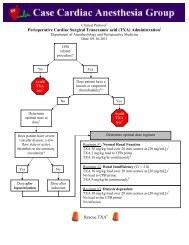
<strong>Clinical</strong> <strong>Protocol</strong> †Cardiac Surgical <strong>Cerebral</strong> <strong>Oximetry</strong> <strong>Clinical</strong> <strong>Protocol</strong> ‡Department of Anesthesiology and Perioperative MedicineDate: 11-07-2011Cardiac surgicalrelated procedure?NoDo notuse thisprotocol 1YesPrepare skin with an alcohol or acetonewipe to optimize sensor placementApply mastisol to skin and allow it todry to a “tacky” state to promoteoptimal sensor adhesionSensors should be carefully placedon the hairless skin of the foreheadtaking care not to allow any hairunder the sensor pads and to avoidplacing it too high on the forehead 2 .Once the sensor pads are placed it is useful to place a long strip of clear1 inch Transpore 3M tape along both the upper and lower borders ofthe pads to promote adhesion.Next, power up the INVOS CPU by pressing the green power buttonlocated on the bottom, left front of the device.
Once powered up you will view the introductory screen shot as shown below. Press the multifunction buttonlocated below the NEW PATIENT option at the bottom of the screen.Next, confirm the ADULT CARDIOVASCULAR database configuration by pressing the white button belowthe YES option.The next screen displayed is the PATIENT IDENTIFIER ENTRY screen. Use this screen to enter thepatient’s Medical Record Number then select DONE.
At this point you should connect the pre-amp module to the sensor pads being careful to insure that CHANNEL1 is the LEFT SIDE and CHANNEL 2 is the RIGHT SIDE. Use the plastic clips (red arrows) to help supportthe cabling to the OR table so as not to put tension on the sensor pads that might peel them off the skin.RLLROnce the device is connected it will display bilateral rSO 2 values 3 and provide an index of signal strength (SSI).At this point it is critical to obtain bilateral ROOM AIR BASELINE values 4 . This is ac<strong>com</strong>plished by selectingthe BASELINE + EVENTS menu as indicated below.This will take you into a submenu that allows you to SET BASELINES.
Once the baselines have been set it is very important to start capturing the Society of Thoracic Surgery (STS)data. This is ac<strong>com</strong>plished by entering the BASELINE + EVENTS menu and selecting INDUCTION. Thiswill start the collection of Area Under the Curve (AUC expressed in min %) data which is captured by ourcenter and reported to the STS. At the end of the case it is imperative to return to the BASELINE + EVENTSmenu and select SKIN CLOSURE to give the AUC an end point for its calculations.<strong>Clinical</strong> Use of <strong>Cerebral</strong> <strong>Oximetry</strong> in Cardiac SurgeryTwo separate clinical strategies are presently accepted related to the use of intraoperative cerebral oximetry incardiac surgical patients.Strategy IIf the baseline rSO 2 values are >50% then one must strive to maintainthe patient’s rSO 2 values no less than25% of the preoperatively establishedroom air baseline.Strategy IIIf the baseline rSO 2 values are ≤50% then one must strive to maintain thepatient’s rSO 2 values ≥ 50% 5 .If a significant cerebral desaturation ٭event is noted then follow the establishedtreatment algorithm presented below.II. Defined٭ as a drop of ≥ 8%, or a drop below a saturation of 50% if using Strategy
<strong>Cerebral</strong> Desaturation Treatment Algorithm1) Assess patient head position to insure that the head has not been inadvertently turned ormoved into any other potential anatomical malposition that may adversely affect cerebral orfacial tissue perfusion.Correct any potential anatomical malposition (e.g., head rotated or steep Trendelenburgposition as the surgical conditions permit)2) Inspect the face to detect plethora, redness or other signs of vascular congestion which mayalso be related to anatomical malposition (e.g., release any pressure on the neck from thesurgical field) and should be corrected as surgical conditions allow.Correct any potential anatomical malposition3) Assess ETCO 2 and/or PaCO 2 .If ETCO 2 or PaCO 2 are < 35 mmHg then adjust mechanical ventilation strategy to allowPaCO2 to climb to a value ≥ 40 mmHg. This can also be done on CPB by adjusting freshgas flow sweep speed in cooperation with the perfusion team.4) Assess mean arterial blood pressure (MAP).If MAP has decreased below 50 mmHg (or below approximately 20% of their baselineMAP) then administer phenylephrine (or other vasoconstrictors as appropriate) to raise theMAP to a value > 60 mmHg or higher as indicated by the patient’s preoperative MAP. Dothis in conjunction with the surgical team so that the pressure is not increased at a timewhen relative hypertension may threaten a surgical <strong>com</strong>plication.5) Assess the CVP to determine if CPB cannula malposition may be impeding jugular venousdrainage or if the position of the heart may be causing a similar phenomenon.If the CVP is > 10 mmHg with a clinical suspicion that one of the above scenarios is theculprit then ask the surgeon to adjust the venous cannula position or reposition the heart asappropriate.6) Assess the cardiac index (CI).If the CI is < 2.0 L/min/m 2 in the setting of what is interpreted to be adequate cerebralperfusion pressure then administer appropriate inotropic support, or work with the perfusionteam if on CPB, to increase the CI to a value > 2.5 L/min/m 2 .7) Assess the FiO 2In patients with persistent and significant desaturations increase the FiO 2 to 100% or a valuethat increases the observed rSO 2 values to a more appropriate level (e.g., within 75% ofroom air baseline or ≥ 50% if employing Strategy II).8) If significant desaturations persist despite the above measures then consider the following:If on CPB, work with the surgical and perfusion teams to reduce the systemictemperature to effect a decrease in CMRO 2If on CPB with an IABP in situ, consider instituting pulsatile perfusion by running theIABP at a fixed rate while on CPBDeepen the anesthetic by administration of additional volatile or intravenous anestheticsto effect a decrease in CMRO 29) Assess the Hemoglobin concentrationIf the hemoglobin concentration is assessed as critically low (e.g., < 7 g/dL) consider earlyadministration of acute normovolemic whole blood if employing an acute normovolemichemodilution (ANH) technique during the case, or administer banked allogeneic red bloodcells to increase the hemoglobin concentration in an effort to improve oxygen carryingcapacity/delivery.
At the end of the case when the skin has been closed always enter the SKIN CLOSURE event as previouslydescribed and alert our perfusion colleagues that the STS data rSO 2 data is ready for harvest.Note that our perfusion colleagues will want the following data for the STS database reporting.Please prompt our perfusion colleagues to collect the STS information when the STS data points are available(i.e. when you have entered the SKIN CLOSURE EVENT). Entering the INDUCTION and SKIN CLOSUREevents can be done at the end of the case if it was not done at the appropriate time points during the case. Toenter these events post hoc simply select the DATABASE SUMMARY menu and follow the prompts. TheSTS data collected includes a dichotomous variable (i.e. YES or NO) of whether the rSO 2 data served as aFIRST ALERT to any intraoperative event that (i.e. technical problem or physiologic change) that couldpotentially lead to an adverse out<strong>com</strong>e. The perfusion team will need your input to accurately answer thisquestion.In almost all cases the sensor pads can be removed from the patient upon OR discharge unless the attendingclinician feels that there is a reason that regional cerebral saturation monitoring should be continued in theCTICU (e.g., ongoing monitoring of the adequacy of frontal lobe oxygen supply related to hemodynamicinstability or a neck hematoma that may impede adequate cerebral perfusion.
†<strong>Clinical</strong> protocols are based on the best and most recent available data and therefore are expected to befollowed by all CASECAG staff unless there is a <strong>com</strong>pelling clinical reason not to (which should bedocumented in the anesthesia record), or new data be<strong>com</strong>es available that suggests an alternate treatmentprotocol. Standardizing the approach to some of our practices is expected to have a positive impact in theclinical environment and improve ease of work flow for the trainees.‡<strong>Cerebral</strong> oximetry use in cardiac surgery has repeatedly demonstrated an improvement in clinical out<strong>com</strong>eswhen studied with prospective, randomized controlled trial and retrospective methodologies (Murkin et al.,Slater et al., Goldman et al.). CASECAG staff and clinicians are expected to employ this technology for eachpatient unless there is an outstanding contraindication to its use (e.g., massive forehead cutaneous trauma).1: This protocol is intended for use in patients undergoing all varieties of cardiac surgical procedures either withor without the use of cardiopulmonary bypass (CPB). While cerebral oximetry has been shown to improveclinical out<strong>com</strong>es in vascular surgical (Botes et al.) and elderly general surgical patients (Casati et al.) thisprotocol is designed to be used specifically on cardiac surgical patients.2: Placement of the sensor pads over hair can result in the phenomenon of “light piping.” Light piping involveshair acting as a fiber optic cable and transmitting ambient near infrared (NIR) light photons into thephotoreceptor and thus disturbing the accuracy of the rSO 2 signal. (Avery EG). Placing the pads too high on theforehead may result in an inaccurate signal if the sensors are located over the cavernous sinus as the pooledvenous blood in this vascular structure will act as a chromophore sink and absorb all of the NIR light emittedphotons, thus preventing the generation of useful rSO 2 data.3: Under most clinical conditions cerebral oximeters will produce a clinical useful rSO 2 value. Pulsatileperfusion is not necessary for the device to produce a useful value. Ambient light contamination is among themost <strong>com</strong>mon reasons that the device will not produce a readable value. (Avery EG) Other reasons that thisdevice will not generate a value include very darkly pigmented skin (e.g., African-American patients), otherunappreciated chromophores in the cutaneous tissue (e.g., collagen) and placing the sensors over darklypigmented hair.4: For a patient that cannot tolerate room air ventilation in the awake state consider obtaining the room airbaseline once the patient is intubated by transiently lowering the FiO 2 to 0.21. Alternatively, in clinicalsituations where a room air baseline cannot be obtained even in an intubated state set your baseline at someFiO 2 consistent with clinical stability.5: Recently published data by Heringlake et al. strongly suggest that cardiac surgical patients with oxygensupplemented room air baseline rSO 2 values ≤ 50% have a significantly higher incidence of postoperativemorbidity and mortality.
References:Murkin JM, Adams SJ, Novick RJ, et al. <strong>Monitoring</strong> Brain Oxygen Saturation during Coronary BypassSurgery: A Randomized Prospective Study. Anesthesia & Analgesia 2007; 104:51-8.Slater JP, Guarino T, Stack J, et al. <strong>Cerebral</strong> Oxygen Desaturation Predicts Cognitive Decline and LongerHospital Stays After Cardiac Sugery. Annals of Thoracic Surgery 2009; 87:36-45.Goldman S, Sutter F, Ferdinand F, et al. Optimizing Intraoperative <strong>Cerebral</strong> Oxygen Delivery UsingNoninvasive <strong>Cerebral</strong> <strong>Oximetry</strong> Decreases the Incidence of Stroke For Cardiac Surgical Patients. The HeartSurgery Forum 2004; 7(5):E376-81.Botes K, Le Roux DA, Van Marle J. <strong>Cerebral</strong> <strong>Monitoring</strong> During Carotid Endarterectomy – A ComparisionBetween Electroencephalography, Transcranial <strong>Cerebral</strong> <strong>Oximetry</strong> and Carotid Stump Pressure. South AfricanJournal of Surgery 2007; 45(2):43-6.Casati A, Fanelli G, Pietropaoli P, et al. Continuous <strong>Monitoring</strong> of <strong>Cerebral</strong> Oxygen Saturation in ElderlyPatients Undergoing Major Abdominal Surgery Minimizes Brain Exposure to Potential Hypoxia. Anesthesia &Analgesia 2005; 101:740-7.Avery EG. <strong>Cerebral</strong> <strong>Oximetry</strong> is Frequently a “First Alert” Indicator of Adverse Out<strong>com</strong>es. White Paper.October 2010. http://www.casecag.<strong>com</strong>/education/Clin%20Util%20Monit%20CerOx_FINAL.pdf last accessed11-08-2011.