Subsurface Iron and Arsenic Removal
qj78kp8
qj78kp8
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1 Introduction<br />
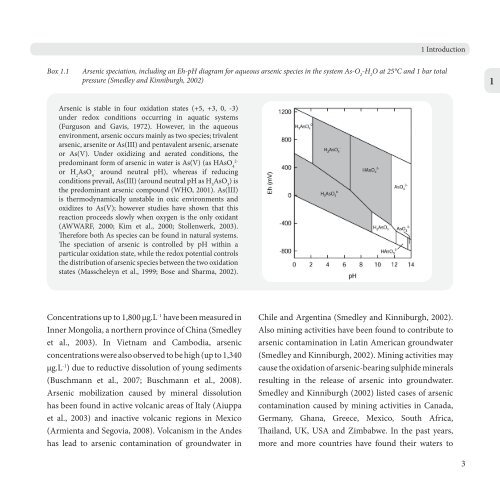
Box 1.1<br />
<strong>Arsenic</strong> speciation, including an Eh-pH diagram for aqueous arsenic species in the system As-O 2<br />
-H 2<br />
O at 25°C <strong>and</strong> 1 bar total<br />
pressure (Smedley <strong>and</strong> Kinniburgh, 2002)<br />
1<br />
<strong>Arsenic</strong> is stable in four oxidation states (+5, +3, 0, -3)<br />
under redox conditions occurring in aquatic systems<br />
(Furguson <strong>and</strong> Gavis, 1972). However, in the aqueous<br />
environment, arsenic occurs mainly as two species; trivalent<br />
arsenic, arsenite or As(III) <strong>and</strong> pentavalent arsenic, arsenate<br />
or As(V). Under oxidizing <strong>and</strong> aerated conditions, the<br />
predominant form of arsenic in water is As(V) (as HAsO 4<br />
2-<br />
or H 2<br />
AsO 4<br />
-<br />
around neutral pH), whereas if reducing<br />
conditions prevail, As(III) (around neutral pH as H 3<br />
AsO 3<br />
) is<br />
the predominant arsenic compound (WHO, 2001). As(III)<br />
is thermodynamically unstable in oxic environments <strong>and</strong><br />
oxidizes to As(V); however studies have shown that this<br />
reaction proceeds slowly when oxygen is the only oxidant<br />
(AWWARF, 2000; Kim et al., 2000; Stollenwerk, 2003).<br />
Therefore both As species can be found in natural systems.<br />
The speciation of arsenic is controlled by pH within a<br />
particular oxidation state, while the redox potential controls<br />
the distribution of arsenic species between the two oxidation<br />
states (Masscheleyn et al., 1999; Bose <strong>and</strong> Sharma, 2002).<br />
Concentrations up to 1,800 μg.L -1 have been measured in<br />
Inner Mongolia, a northern province of China (Smedley<br />
et al., 2003). In Vietnam <strong>and</strong> Cambodia, arsenic<br />
concentrations were also observed to be high (up to 1,340<br />
μg.L -1 ) due to reductive dissolution of young sediments<br />
(Buschmann et al., 2007; Buschmann et al., 2008).<br />
<strong>Arsenic</strong> mobilization caused by mineral dissolution<br />
has been found in active volcanic areas of Italy (Aiuppa<br />
et al., 2003) <strong>and</strong> inactive volcanic regions in Mexico<br />
(Armienta <strong>and</strong> Segovia, 2008). Volcanism in the Andes<br />
has lead to arsenic contamination of groundwater in<br />
Chile <strong>and</strong> Argentina (Smedley <strong>and</strong> Kinniburgh, 2002).<br />
Also mining activities have been found to contribute to<br />
arsenic contamination in Latin American groundwater<br />
(Smedley <strong>and</strong> Kinniburgh, 2002). Mining activities may<br />
cause the oxidation of arsenic-bearing sulphide minerals<br />
resulting in the release of arsenic into groundwater.<br />
Smedley <strong>and</strong> Kinniburgh (2002) listed cases of arsenic<br />
contamination caused by mining activities in Canada,<br />
Germany, Ghana, Greece, Mexico, South Africa,<br />
Thail<strong>and</strong>, UK, USA <strong>and</strong> Zimbabwe. In the past years,<br />
more <strong>and</strong> more countries have found their waters to<br />
3