Subsurface Iron and Arsenic Removal
qj78kp8
qj78kp8
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
6 Characterization of accumulated deposits<br />
%mol ratio was on average 4.8 (±1.65) <strong>and</strong> 5.4 (±1.26)<br />
near well 04 <strong>and</strong> the reference well, respectively. The<br />
precipitates in Figure 6.7(c) <strong>and</strong> (d) have been found<br />
in the deeper layer at 23-24m. ESEM-EDX showed<br />
that the Ca:S ratio in coating (c) is 0.85, which roughly<br />
corresponds to CaSO 4<br />
(gypsum). Calcium sulfate has<br />
not been observed in any of the samples taken between<br />
19 <strong>and</strong> 22m depth, neither near well 04 nor in the<br />
reference well. The same was the case for the occurrence<br />
of the framboidal pyrite (FeS 2<br />
, (d)). In the presence of<br />
oxygen, pyrite is known to oxidize <strong>and</strong> release sulphate<br />
<strong>and</strong> iron into the groundwater. In addition, pyrite<br />
oxidation is an acidifying reaction <strong>and</strong> therefore not<br />
promoting the (rapid) oxidation of Fe 2+ . The presence<br />
of sulphide minerals is a vital difference between the<br />
depths with iron accumulation (19-22m) <strong>and</strong> without<br />
iron accumulation (deeper than 22m).<br />
X-Ray Powder Diffraction <strong>and</strong> 57 Fe<br />
Mössbauer Spectroscopy<br />
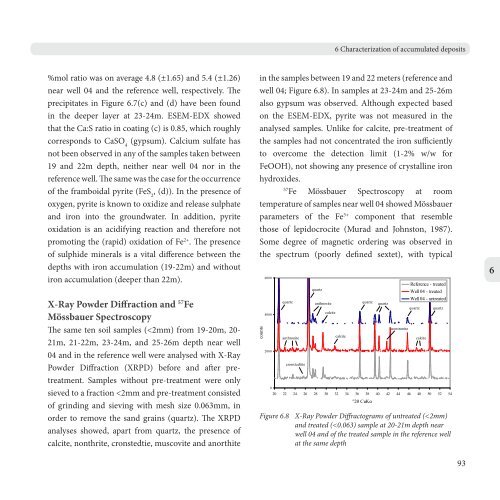
The same ten soil samples (