Subsurface Iron and Arsenic Removal
qj78kp8
qj78kp8
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
6 Characterization of accumulated deposits<br />
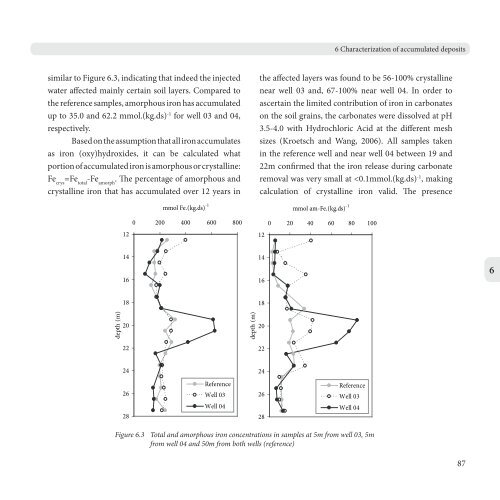
similar to Figure 6.3, indicating that indeed the injected<br />
water affected mainly certain soil layers. Compared to<br />
the reference samples, amorphous iron has accumulated<br />
up to 35.0 <strong>and</strong> 62.2 mmol.(kg.ds) -1 for well 03 <strong>and</strong> 04,<br />
respectively.<br />
Based on the assumption that all iron accumulates<br />
as iron (oxy)hydroxides, it can be calculated what<br />
portion of accumulated iron is amorphous or crystalline:<br />
Fe crys<br />
=Fe total<br />
-Fe amorph<br />
. The percentage of amorphous <strong>and</strong><br />
crystalline iron that has accumulated over 12 years in<br />
12<br />
mmol Fe.(kg.ds) -1<br />
0 200 400 600 800<br />
the affected layers was found to be 56-100% crystalline<br />
near well 03 <strong>and</strong>, 67-100% near well 04. In order to<br />
ascertain the limited contribution of iron in carbonates<br />
on the soil grains, the carbonates were dissolved at pH<br />
3.5-4.0 with Hydrochloric Acid at the different mesh<br />
sizes (Kroetsch <strong>and</strong> Wang, 2006). All samples taken<br />
in the reference well <strong>and</strong> near well 04 between 19 <strong>and</strong><br />
22m confirmed that the iron release during carbonate<br />
removal was very small at