Subsurface Iron and Arsenic Removal
qj78kp8
qj78kp8
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Subsurface</strong> iron <strong>and</strong> arsenic removal for drinking water treatment in Bangladesh<br />
2<br />
[PO4 3- ] mmol.L -1<br />
0.05<br />
0.04<br />
0.03<br />
0.02<br />
0.01<br />
0.00<br />
Cycle 1<br />
Cycle 7<br />
Cycle 22<br />
0 5 10 15<br />
V/Vi<br />
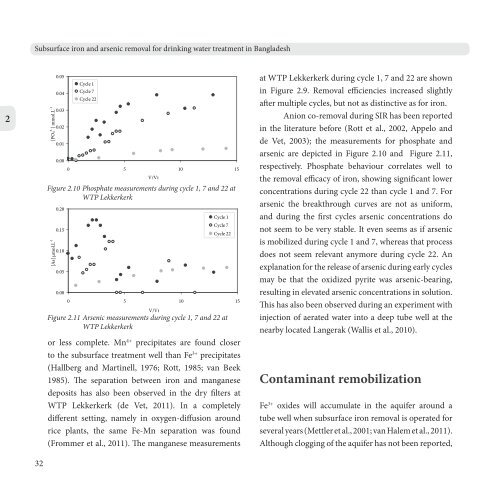
Figure 2.10 Phosphate measurements during cycle 1, 7 <strong>and</strong> 22 at<br />
WTP Lekkerkerk<br />
[As] µmol.L -1<br />
0.20<br />
0.15<br />
0.10<br />
0.05<br />
0.00<br />
0 5 10 15<br />
V/Vi<br />
Cycle 1<br />
Cycle 7<br />
Cycle 22<br />
Figure 2.11 <strong>Arsenic</strong> measurements during cycle 1, 7 <strong>and</strong> 22 at<br />
WTP Lekkerkerk<br />
or less complete. Mn 4+ precipitates are found closer<br />
to the subsurface treatment well than Fe 3+ precipitates<br />
(Hallberg <strong>and</strong> Martinell, 1976; Rott, 1985; van Beek<br />
1985). The separation between iron <strong>and</strong> manganese<br />
deposits has also been observed in the dry filters at<br />
WTP Lekkerkerk (de Vet, 2011). In a completely<br />
different setting, namely in oxygen-diffusion around<br />
rice plants, the same Fe-Mn separation was found<br />
(Frommer et al., 2011). The manganese measurements<br />
at WTP Lekkerkerk during cycle 1, 7 <strong>and</strong> 22 are shown<br />
in Figure 2.9. <strong>Removal</strong> efficiencies increased slightly<br />
after multiple cycles, but not as distinctive as for iron.<br />
Anion co-removal during SIR has been reported<br />
in the literature before (Rott et al., 2002, Appelo <strong>and</strong><br />
de Vet, 2003); the measurements for phosphate <strong>and</strong><br />
arsenic are depicted in Figure 2.10 <strong>and</strong> Figure 2.11,<br />
respectively. Phosphate behaviour correlates well to<br />
the removal efficacy of iron, showing significant lower<br />
concentrations during cycle 22 than cycle 1 <strong>and</strong> 7. For<br />
arsenic the breakthrough curves are not as uniform,<br />
<strong>and</strong> during the first cycles arsenic concentrations do<br />
not seem to be very stable. It even seems as if arsenic<br />
is mobilized during cycle 1 <strong>and</strong> 7, whereas that process<br />
does not seem relevant anymore during cycle 22. An<br />
explanation for the release of arsenic during early cycles<br />
may be that the oxidized pyrite was arsenic-bearing,<br />
resulting in elevated arsenic concentrations in solution.<br />
This has also been observed during an experiment with<br />
injection of aerated water into a deep tube well at the<br />
nearby located Langerak (Wallis et al., 2010).<br />
Contaminant remobilization<br />
Fe 3+ oxides will accumulate in the aquifer around a<br />
tube well when subsurface iron removal is operated for<br />
several years (Mettler et al., 2001; van Halem et al., 2011).<br />
Although clogging of the aquifer has not been reported,<br />
32