Chemical Potential, Fugacity, Ideal Solutions, Activity, and Excess ...
Chemical Potential, Fugacity, Ideal Solutions, Activity, and Excess ...
Chemical Potential, Fugacity, Ideal Solutions, Activity, and Excess ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Chemical</strong> <strong>Potential</strong>, <strong>Fugacity</strong>, <strong>Ideal</strong> <strong>Solutions</strong>, <strong>Activity</strong>, <strong>and</strong> <strong>Excess</strong><br />
Gibbs Energy<br />
Prof. Geof Silcox<br />
<strong>Chemical</strong> Engineering Thermodynamics (CH EN 3853)<br />
University of Utah<br />
2012 June 15<br />
1.0 Mixtures of <strong>Ideal</strong> Gases, the Gibbs Energy, <strong>and</strong> the <strong>Chemical</strong> <strong>Potential</strong><br />
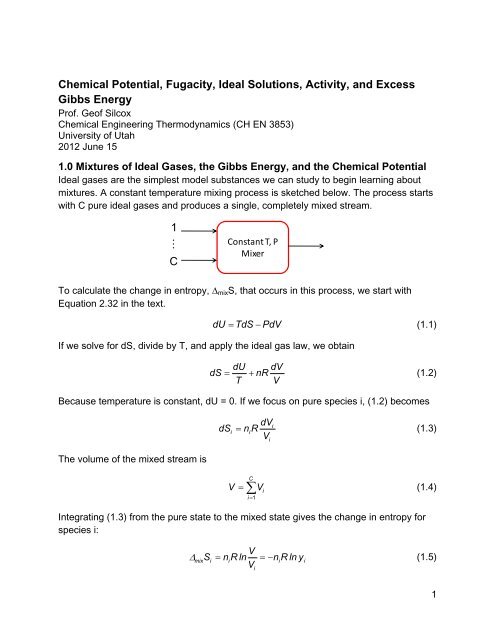
<strong>Ideal</strong> gases are the simplest model substances we can study to begin learning about<br />
mixtures. A constant temperature mixing process is sketched below. The process starts<br />
with C pure ideal gases <strong>and</strong> produces a single, completely mixed stream.<br />
To calculate the change in entropy, �mixS, that occurs in this process, we start with<br />
Equation 2.32 in the text.<br />
dU � TdS � PdV<br />
(1.1)<br />
If we solve for dS, divide by T, <strong>and</strong> apply the ideal gas law, we obtain<br />
dU dV<br />
dS � � nR<br />
(1.2)<br />
T V<br />
Because temperature is constant, dU = 0. If we focus on pure species i, (1.2) becomes<br />
The volume of the mixed stream is<br />
1<br />
�<br />
C<br />
Constant T, P<br />
Mixer<br />
dV<br />
dS n R<br />
V<br />
i � i<br />
i<br />
(1.3)<br />
i<br />
C<br />
V � � Vi<br />
Integrating (1.3) from the pure state to the mixed state gives the change in entropy for<br />
species i:<br />
i �1<br />
mix i i i i<br />
Vi<br />
(1.4)<br />
V<br />
� S � nRln � � nRlny<br />
(1.5)<br />
1
According to (1.5) the entropy change of mixing is always a positive number. The<br />
change in entropy for all species summed together is<br />
or<br />
ig<br />
mixS ig<br />
S<br />
C<br />
ig<br />
ns i i R<br />
C<br />
niln yi<br />
i�1 i�1<br />
� � � � �<br />
The corresponding change in enthalpy is<br />
or<br />
Since G = H – TS, it follows that<br />
� � (1.6)<br />
ig<br />
�<br />
C<br />
i<br />
ig<br />
i �<br />
C<br />
i ln i<br />
i�1 i�1<br />
� � (1.7)<br />
S ns R n y<br />
C<br />
ig ig ig<br />
mixH H nh i i<br />
i �1<br />
� � �� �0<br />
(1.8)<br />
C<br />
ig ig<br />
i i<br />
i �1<br />
H � � nh<br />
(1.9)<br />
ig<br />
�<br />
C<br />
i<br />
ig<br />
i �<br />
C<br />
i ln i<br />
i�1 i�1<br />
� � (1.10)<br />
g y g RT y y<br />
The partial molar equation (6.5, p. 74 of the text), written for the ideal gas Gibbs energy,<br />
is<br />
Comparison of (1.10) <strong>and</strong> (1.11) gives<br />
ig<br />
�<br />
C<br />
i<br />
ig<br />
i �<br />
C<br />
i<br />
ig<br />
i<br />
i�1 i�1<br />
� � (1.11)<br />
g y g y �<br />
ig ig ig<br />
g � � � g � RTln y<br />
(1.12)<br />
i i i i<br />
Note that (1.12) implies that as the mole fraction, yi, approaches zero,<br />
minus infinity.<br />
Recall that (1.12) is at pressure P. It is convenient to define<br />
different pressure, P 0 . For pure i,<br />
ig<br />
� i relative to<br />
ig<br />
� i approaches<br />
ig<br />
g i at a<br />
dgi � �sidT� vidP (1.13)<br />
2
For a pure ideal gas, <strong>and</strong> holding T constant, (1.13) is<br />
Integration of (1.14) from P 0 to P gives<br />
ig 0<br />
where i � �<br />
dg �vdP� RTd P<br />
(1.14)<br />
ig ig<br />
iT , i ln<br />
ig ig 0 P<br />
gi �P��gi �P � � RTln (1.15)<br />
0<br />
P<br />
g P is a function of temperature alone. Substituting (1.12) in (1.15) gives<br />
ig 0<br />
Note that i � �<br />
ig ig 0 �yiP� �i<br />
�gi �P , T��RTln� o<br />
P<br />
�<br />
� � (1.16)<br />
g P ,T is independent of composition but is different for every ideal gas. A<br />
convenient choice for P 0 is 1 bar whence (1.16) becomes<br />
�<br />
� y P �<br />
�g �1 bar, T��RTln� 1 bar<br />
�<br />
� � (1.17)<br />
ig ig i<br />
i i<br />
For simplicity, we will not carry the 1 bar that appears in (1.17). For a pure ideal gas,<br />
(1.17) becomes<br />
ig ig<br />
i i<br />
� � ln�<br />
�<br />
� � g T � RT P<br />
(1.18)<br />
2.0 <strong>Fugacity</strong><br />
The definition of fugacity is chosen to make (1.17) valid for a real mixture of gases or<br />
liquids:<br />
ig<br />
� �g ( T) � RTlnf (2.1)<br />
i i i<br />
This is (7.1), p. 89 of your text. For a pure substance, (2.1) becomes<br />
ig o<br />
g � g ( T) � RTlnf (2.2)<br />
i i i<br />
The mixture fugacity coefficient, ˆ � i , is a dimensionless property defined by subtracting<br />
(1.17) from (2.1) to give<br />
<strong>and</strong><br />
ig fi<br />
ig<br />
� ln ln ˆ<br />
i � �i �RT � �i � RT �i<br />
(2.3)<br />
yP<br />
i<br />
3
For a pure substance, subtracting (1.18) from (2.2) gives<br />
where the pure species fugacity coefficient is<br />
As P approaches zero, (2.4) <strong>and</strong> (2.6) approach one.<br />
Equation (2.2) can be subtracted from (2.1) to give<br />
ˆ<br />
fi<br />
�i � (2.4)<br />
y P<br />
i<br />
o<br />
ig fi<br />
gi � gi � RTln (2.5)<br />
P<br />
o<br />
fi<br />
�i � (2.6)<br />
P<br />
ln i f<br />
� � g � RT (2.7)<br />
f<br />
i i o<br />
i<br />
where / o<br />
fi fi � ai<br />
is called the activity. The activity is useful in the treatment of chemical<br />
equilibrium.<br />
3.0 <strong>Ideal</strong> <strong>Solutions</strong><br />
<strong>Ideal</strong> solutions are defined as solutions for which<br />
is<br />
�<br />
C<br />
i<br />
is<br />
i <strong>and</strong> v �<br />
C<br />
i i<br />
i�1 i�1<br />
� � (3.1)<br />
h x h xv<br />
From the discussion above of mixtures of ideal gases, <strong>and</strong> by analogy with (1.10),<br />
is<br />
g �<br />
C<br />
xigi �RT<br />
C<br />
xi ln xi<br />
i�1 i�1<br />
� � (3.2)<br />
where gi is the Gibbs energy, kJ/mol, of pure i. For a liquid or solid solution, gi is the<br />
Gibbs energy of the pure liquid or solid. The use of gi for the liquid or solid distinguishes<br />
(3.2) from (1.10).<br />
From (1.11) <strong>and</strong> (3.2),<br />
4.0 <strong>Excess</strong> Gibbs Energy<br />
The excess Gibbs energy is defined by<br />
is<br />
� � g � RTln x<br />
(3.3)<br />
i i i<br />
4
The activity coefficient is defined by<br />
Comparison of (4.2) with (1.11) shows that<br />
E � �<br />
�G<br />
� �<br />
� �n<br />
�<br />
E is<br />
g � g � g<br />
(4.1)<br />
C<br />
E<br />
g � RT� x ln�<br />
(4.2)<br />
i TPn , , j�i From (3.2), (4.1), <strong>and</strong> (4.2) it follows that<br />
i �1<br />
i i<br />
E E<br />
�g � � �RTln�<br />
i i i<br />
is E<br />
g � g �g �<br />
C<br />
xigi �RT C<br />
xi ln xi �RT<br />
C<br />
xi ln�<br />
i<br />
i�1 i�1 i�1<br />
Comparison of (4.4) with (1.11) shows that<br />
Subtracting (2.2) from (2.1) yields<br />
Comparison of (4.5) <strong>and</strong> (4.6) shows that<br />
(4.3)<br />
� � � (4.4)<br />
� � g � RTln x�<br />
(4.5)<br />
i i i i<br />
ln i f<br />
� � g � RT (4.6)<br />
f<br />
i i o<br />
i<br />
f a<br />
� � � (4.7)<br />
i i<br />
i o<br />
xifixi where ai is the activity <strong>and</strong> �i is the activity coefficient. For an ideal solution, ai � xi,<br />
f � xf , <strong>and</strong> � � 1.<br />
o<br />
i i i<br />
i<br />
5