March 2019 digital v1
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
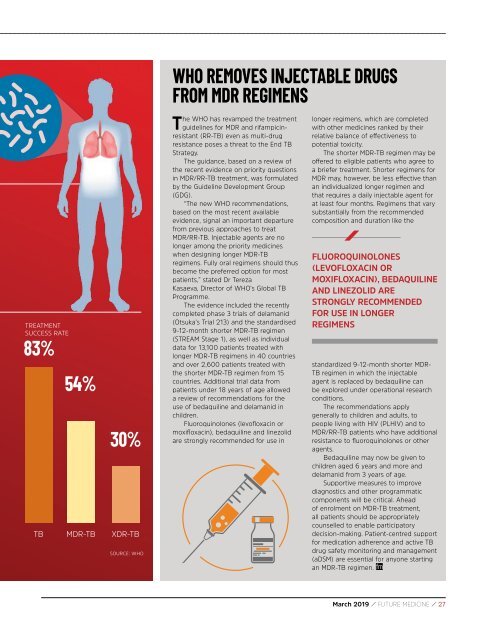
TREATMENT<br />
SUCCESS RATE<br />
83%<br />
54%<br />
30%<br />
TB MDR-TB XDR-TB<br />
SOURCE: WHO<br />
WHO REMOVES INJECTABLE DRUGS<br />
FROM MDR REGIMENS<br />
The WHO has revamped the treatment<br />
guidelines for MDR and rifampicinresistant<br />
(RR-TB) even as multi-drug<br />
resistance poses a threat to the End TB<br />
Strategy.<br />
The guidance, based on a review of<br />
the recent evidence on priority questions<br />
in MDR/RR-TB treatment, was formulated<br />
by the Guideline Development Group<br />
(GDG).<br />
“The new WHO recommendations,<br />
based on the most recent available<br />
evidence, signal an important departure<br />
from previous approaches to treat<br />
MDR/RR-TB. Injectable agents are no<br />
longer among the priority medicines<br />
when designing longer MDR-TB<br />
regimens. Fully oral regimens should thus<br />
become the preferred option for most<br />
patients,” stated Dr Tereza<br />
Kasaeva, Director of WHO’s Global TB<br />
Programme.<br />
The evidence included the recently<br />
completed phase 3 trials of delamanid<br />
(Otsuka’s Trial 213) and the standardised<br />
9-12-month shorter MDR-TB regimen<br />
(STREAM Stage 1), as well as individual<br />
data for 13,100 patients treated with<br />
longer MDR-TB regimens in 40 countries<br />
and over 2,600 patients treated with<br />
the shorter MDR-TB regimen from 15<br />
countries. Additional trial data from<br />
patients under 18 years of age allowed<br />
a review of recommendations for the<br />
use of bedaquiline and delamanid in<br />
children.<br />
Fluoroquinolones (levofloxacin or<br />
moxifloxacin), bedaquiline and linezolid<br />
are strongly recommended for use in<br />
longer regimens, which are completed<br />
with other medicines ranked by their<br />
relative balance of effectiveness to<br />
potential toxicity.<br />
The shorter MDR-TB regimen may be<br />
offered to eligible patients who agree to<br />
a briefer treatment. Shorter regimens for<br />
MDR may, however, be less effective than<br />
an individualized longer regimen and<br />
that requires a daily injectable agent for<br />
at least four months. Regimens that vary<br />
substantially from the recommended<br />
composition and duration like the<br />
FLUOROQUINOLONES<br />
(LEVOFLOXACIN OR<br />
MOXIFLOXACIN), BEDAQUILINE<br />
AND LINEZOLID ARE<br />
STRONGLY RECOMMENDED<br />
FOR USE IN LONGER<br />
REGIMENS<br />
standardized 9-12-month shorter MDR-<br />
TB regimen in which the injectable<br />
agent is replaced by bedaquiline can<br />
be explored under operational research<br />
conditions.<br />
The recommendations apply<br />
generally to children and adults, to<br />
people living with HIV (PLHIV) and to<br />
MDR/RR-TB patients who have additional<br />
resistance to fluoroquinolones or other<br />
agents.<br />
Bedaquiline may now be given to<br />
children aged 6 years and more and<br />
delamanid from 3 years of age.<br />
Supportive measures to improve<br />
diagnostics and other programmatic<br />
components will be critical. Ahead<br />
of enrolment on MDR-TB treatment,<br />
all patients should be appropriately<br />
counselled to enable participatory<br />
decision-making. Patient-centred support<br />
for medication adherence and active TB<br />
drug safety monitoring and management<br />
(aDSM) are essential for anyone starting<br />
an MDR-TB regimen.<br />
<strong>March</strong> <strong>2019</strong> / FUTURE MEDICINE / 27