2008 Scientific Report
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
VARI | <strong>2008</strong><br />
Research Interests<br />
The primary focus of the Systems Biology laboratory is identifying and understanding the genes and signaling pathways that,<br />
when mutated, contribute to the pathophysiology of cancer. We take advantage of RNA interference (RNAi) and novel proteomic<br />
approaches to identify the enzymes that control cell growth, proliferation, and survival. For example, after screening the human<br />
genome for more than 600 kinases and 200 phosphatases—called the “kinome” and “phosphatome”, respectively—that act<br />
with chemotherapeutic agents in controlling apoptosis, we identified several essential kinases and phosphatases whose roles<br />
in cell survival were previously unrecognized. We are asking several questions. How are these survival enzymes regulated at<br />
the molecular level? What signaling pathway(s) do they regulate? Does changing the number of enzyme molecules present<br />
inhibit waves of compensatory changes at the cellular level (system-level changes)? What are the system-level changes after<br />
reduction or loss of each gene?<br />
Novel modulators of chemotherapeutic sensitization<br />
Kinases and phosphatases play an integral role in balancing the survival and apoptotic signals within a cell. In an attempt to<br />
define proteins with a major role in these processes, we tested an RNAi library against all known kinases and phosphatases<br />
in the human genome and assayed various phenotypes, including sensitization to apoptosis and chemoresistance. A group<br />
of apoptosis sensitizers was identified whose siRNA knock-out conferred a marked increase in cell survival as well as a<br />
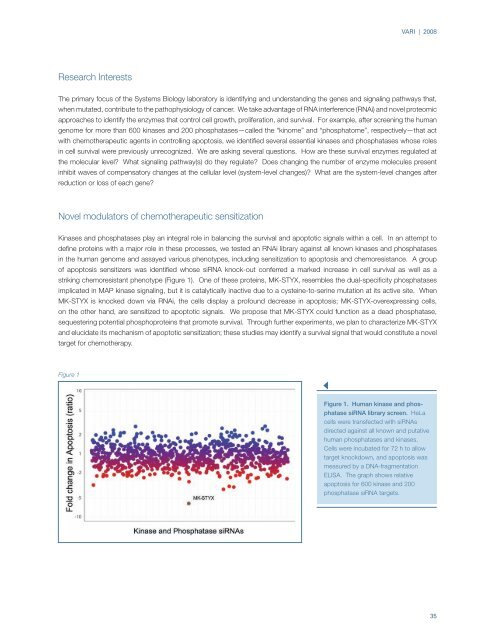
striking chemoresistant phenotype (Figure 1). One of these proteins, MK-STYX, resembles the dual-specificity phosphatases<br />
implicated in MAP kinase signaling, but it is catalytically inactive due to a cysteine-to-serine mutation at its active site. When<br />
MK-STYX is knocked down via RNAi, the cells display a profound decrease in apoptosis; MK-STYX-overexpressing cells,<br />
on the other hand, are sensitized to apoptotic signals. We propose that MK-STYX could function as a dead phosphatase,<br />
sequestering potential phosphoproteins that promote survival. Through further experiments, we plan to characterize MK-STYX<br />
and elucidate its mechanism of apoptotic sensitization; these studies may identify a survival signal that would constitute a novel<br />
target for chemotherapy.<br />
Figure 1<br />
Figure 1. Human kinase and phosphatase<br />
siRNA library screen. HeLa<br />
cells were transfected with siRNAs<br />
directed against all known and putative<br />
human phosphatases and kinases.<br />
Cells were incubated for 72 h to allow<br />
target knockdown, and apoptosis was<br />
measured by a DNA-fragmentation<br />
ELISA. The graph shows relative<br />
apoptosis for 600 kinase and 200<br />
phosphatase siRNA targets.<br />
35