Aurobindo Pharma - The Smart Investor
Aurobindo Pharma - The Smart Investor
Aurobindo Pharma - The Smart Investor
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
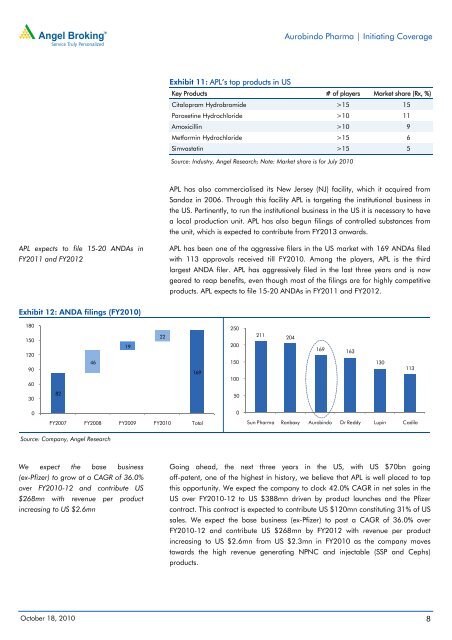
APL expects to file 15-20 ANDAs in<br />
FY2011 and FY2012<br />
Exhibit 12: ANDA filings (FY2010)<br />
180<br />
150<br />
120<br />
90<br />
60<br />
30<br />
0<br />
82<br />
46<br />
Source: Company, Angel Research<br />
19<br />
22<br />
Exhibit 11: APL’s top products in US<br />
<strong>Aurobindo</strong> <strong>Pharma</strong> | Initiating Coverage<br />
Key Products # of players Market share (Rx, %)<br />
Citalopram Hydrobromide >15 15<br />
Paroxetine Hydrochloride >10 11<br />
Amoxicillin >10 9<br />
Metformin Hydrochloride >15 6<br />
Simvastatin >15 5<br />
Source: Industry, Angel Research; Note: Market share is for July 2010<br />
APL has also commercialised its New Jersey (NJ) facility, which it acquired from<br />
Sandoz in 2006. Through this facility APL is targeting the institutional business in<br />
the US. Pertinently, to run the institutional business in the US it is necessary to have<br />
a local production unit. APL has also begun filings of controlled substances from<br />
the unit, which is expected to contribute from FY2013 onwards.<br />
APL has been one of the aggressive filers in the US market with 169 ANDAs filed<br />
with 113 approvals received till FY2010. Among the players, APL is the third<br />
largest ANDA filer. APL has aggressively filed in the last three years and is now<br />
geared to reap benefits, even though most of the filings are for highly competitive<br />
products. APL expects to file 15-20 ANDAs in FY2011 and FY2012.<br />
169<br />
FY2007 FY2008 FY2009 FY2010 Total<br />
We expect the base business<br />
(ex-Pfizer) to grow at a CAGR of 36.0%<br />
over FY2010-12 and contribute US<br />
$268mn with revenue per product<br />
increasing to US $2.6mn<br />
Going ahead, the next three years in the US, with US $70bn going<br />
off-patent, one of the highest in history, we believe that APL is well placed to tap<br />
this opportunity. We expect the company to clock 42.0% CAGR in net sales in the<br />
US over FY2010-12 to US $388mn driven by product launches and the Pfizer<br />
contract. This contract is expected to contribute US $120mn constituting 31% of US<br />
sales. We expect the base business (ex-Pfizer) to post a CAGR of 36.0% over<br />
FY2010-12 and contribute US $268mn by FY2012 with revenue per product<br />
increasing to US $2.6mn from US $2.3mn in FY2010 as the company moves<br />
towards the high revenue generating NPNC and injectable (SSP and Cephs)<br />
products.<br />
October 18, 2010 8<br />
250<br />
200<br />
150<br />
100<br />
50<br />
0<br />
211<br />
204<br />
169 163<br />
130<br />
113<br />
Sun <strong>Pharma</strong> Ranbaxy <strong>Aurobindo</strong> Dr Reddy Lupin Cadila