Surimi wash water treatment by chitosan-alginate complexes
Surimi wash water treatment by chitosan-alginate complexes
Surimi wash water treatment by chitosan-alginate complexes
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Muraki and others (1993). In this method, acetic acid solutions, glucosamine<br />
standards and the test sample are scanned in the UV region to obtain first<br />
derivatives of each spectrum. Acetic acid solutions (0.01, 0.02, and 0.03 M) are<br />
used to construct the zero crossing point (ZCP) which is the point where acetic acid<br />
does not interfere with the sample spectra. The point in which the spectrum passes<br />
through the ZCP is used to determine the base line. A series of standard solution of<br />
N-acetyl-D-glucosamine (GlcNAc) in 0.01 M acetic acid is used to prepare a<br />
standard calibration curve. The curve is a plot of GlcNAc concentration versus the<br />
vertical distance of each peak of the first derivative spectra to the base line (ZCP).<br />
First derivative UV-adsorption of unknown sample in 0.01 M acetic acid is then<br />
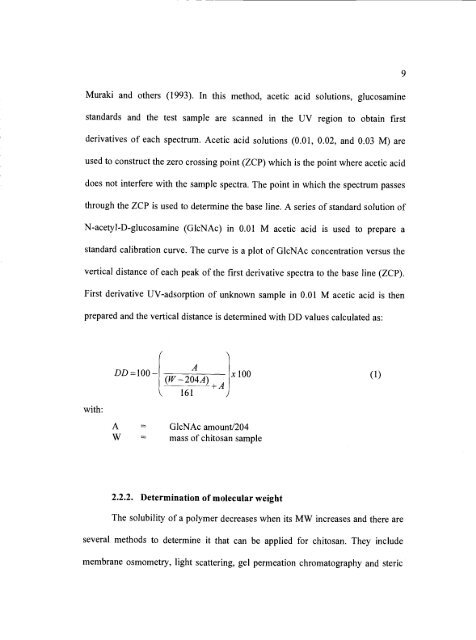
prepared and the vertical distance is determined with DD values calculated as:<br />
with:<br />
A<br />
(W-204A)<br />
DD=100_[______ xlOO (1)<br />
+A1<br />
161 )<br />
A = G1cNAc amount/204<br />
W = mass of <strong>chitosan</strong> sample<br />
2.2.2. Determination of molecular weight<br />
The solubility of a polymer decreases when its MW increases and there are<br />
several methods to determine it that can be applied for <strong>chitosan</strong>. They include<br />
membrane osmometry, light scattering, gel permeation chromatography and steric