Sirtex Cover.proof 11 - School of Educators
Sirtex Cover.proof 11 - School of Educators
Sirtex Cover.proof 11 - School of Educators
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
5Section<br />
16<br />
Overview <strong>of</strong> <strong>Sirtex</strong> Medical<br />
(ASCO) on 16 May, 1999 and caused great<br />
interest. <strong>Sirtex</strong> Medical’s presentation at<br />
ASCO was one <strong>of</strong> a small group <strong>of</strong> new<br />
studies that was selected out <strong>of</strong> more than<br />
2000 presentations by the ASCO Committee<br />
for a news conference on ‘New Technologies<br />
for the Future’.<br />
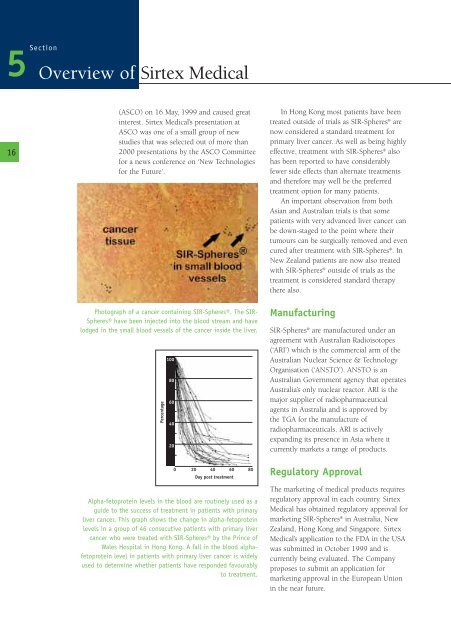
Photograph <strong>of</strong> a cancer containing SIR-Spheres®. The SIR-<br />
Spheres® have been injected into the blood stream and have<br />
lodged in the small blood vessels <strong>of</strong> the cancer inside the liver.<br />
Percentage<br />
100<br />
80<br />
60<br />
40<br />
20<br />
0 20 40 60 80<br />
Day post treatment<br />
Alpha-fetoprotein levels in the blood are routinely used as a<br />
guide to the success <strong>of</strong> treatment in patients with primary<br />
liver cancer. This graph shows the change in alpha-fetoprotein<br />
levels in a group <strong>of</strong> 46 consecutive patients with primary liver<br />
cancer who were treated with SIR-Spheres® by the Prince <strong>of</strong><br />
Wales Hospital in Hong Kong. A fall in the blood alphafetoprotein<br />
level in patients with primary liver cancer is widely<br />
used to determine whether patients have responded favourably<br />
to treatment.<br />
In Hong Kong most patients have been<br />
treated outside <strong>of</strong> trials as SIR-Spheres ® are<br />
now considered a standard treatment for<br />
primary liver cancer. As well as being highly<br />
effective, treatment with SIR-Spheres ® also<br />
has been reported to have considerably<br />
fewer side effects than alternate treatments<br />
and therefore may well be the preferred<br />
treatment option for many patients.<br />
An important observation from both<br />
Asian and Australian trials is that some<br />
patients with very advanced liver cancer can<br />
be down-staged to the point where their<br />
tumours can be surgically removed and even<br />
cured after treatment with SIR-Spheres ® . In<br />
New Zealand patients are now also treated<br />
with SIR-Spheres ® outside <strong>of</strong> trials as the<br />
treatment is considered standard therapy<br />
there also.<br />
Manufacturing<br />
SIR-Spheres ® are manufactured under an<br />
agreement with Australian Radioisotopes<br />
(‘ARI’) which is the commercial arm <strong>of</strong> the<br />
Australian Nuclear Science & Technology<br />
Organisation (‘ANSTO’). ANSTO is an<br />
Australian Government agency that operates<br />
Australia’s only nuclear reactor. ARI is the<br />
major supplier <strong>of</strong> radiopharmaceutical<br />
agents in Australia and is approved by<br />
the TGA for the manufacture <strong>of</strong><br />
radiopharmaceuticals. ARI is actively<br />
expanding its presence in Asia where it<br />
currently markets a range <strong>of</strong> products.<br />
Regulatory Approval<br />
The marketing <strong>of</strong> medical products requires<br />
regulatory approval in each country. <strong>Sirtex</strong><br />
Medical has obtained regulatory approval for<br />
marketing SIR-Spheres ® in Australia, New<br />
Zealand, Hong Kong and Singapore. <strong>Sirtex</strong><br />
Medical’s application to the FDA in the USA<br />
was submitted in October 1999 and is<br />
currently being evaluated. The Company<br />
proposes to submit an application for<br />
marketing approval in the European Union<br />
in the near future.