Download the entire issue - American Association for Clinical ...
Download the entire issue - American Association for Clinical ...
Download the entire issue - American Association for Clinical ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
care. Un<strong>for</strong>tunately, most laboratories are<br />
not able to do this because archived unused<br />
specimens usually are not available.<br />
Setting <strong>the</strong> Reference Range<br />
Biochemical test results are typically reported<br />
relative to a reference range established<br />
from measurements made on individuals<br />
without conditions likely to affect<br />
<strong>the</strong> test. Any Tg assay reference range established<br />
using normal euthyroid subjects will<br />
be influenced by <strong>the</strong> rigor used to exclude<br />
individuals with thyroid pathologies such<br />
as goiter and thyroiditis. Regardless of <strong>the</strong>se<br />
factors, Tg reference ranges established <strong>for</strong><br />
normal euthyroid subjects have little relevance<br />
when interpreting serum Tg concentrations<br />
in thyroidectomized DTC patients.<br />
In <strong>the</strong>se patients, it is better to interpret<br />
serum Tg levels relative to <strong>the</strong> degree of<br />
surgery (lobectomy versus near-total thyroidectomy),<br />
<strong>the</strong> TSH status of <strong>the</strong> patient,<br />
and <strong>the</strong> technical benchmarks of <strong>the</strong> assay<br />
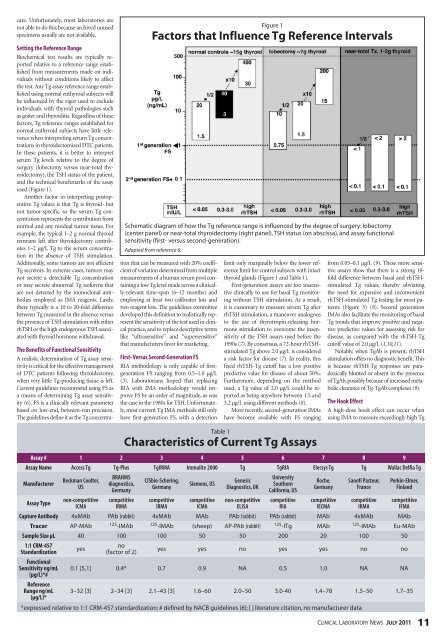
used (Figure 1).<br />
Ano<strong>the</strong>r factor in interpreting postoperative<br />
Tg values is that Tg is thyroid- but<br />
not tumor-specific, so <strong>the</strong> serum Tg concentration<br />
represents <strong>the</strong> contribution from<br />
normal and any residual tumor t<strong>issue</strong>. For<br />
example, <strong>the</strong> typical 1–2 g normal thyroid<br />
remnant left after thyroidectomy contributes<br />
1–2 µg/L Tg to <strong>the</strong> serum concentration<br />
in <strong>the</strong> absence of TSH stimulation.<br />
Additionally, some tumors are not efficient<br />
Tg secretors. In extreme cases, tumors may<br />
not secrete a detectable Tg concentration<br />
or may secrete abnormal Tg iso<strong>for</strong>ms that<br />
are not detected by <strong>the</strong> monoclonal antibodies<br />
employed as IMA reagents. Lastly,<br />
<strong>the</strong>re typically is a 10 to 20-fold difference<br />
between Tg measured in <strong>the</strong> absence versus<br />
<strong>the</strong> presence of TSH stimulation with ei<strong>the</strong>r<br />
rhTSH or <strong>the</strong> high endogenous TSH associated<br />
with thyroid hormone withdrawal.<br />
The Benefits of Functional Sensitivity<br />
A realistic determination of Tg assay sensitivity<br />
is critical <strong>for</strong> <strong>the</strong> effective management<br />
of DTC patients following thyroidectomy,<br />
when very little Tg-producing t<strong>issue</strong> is left.<br />
Current guidelines recommend using FS as<br />
a means of determining Tg assay sensitivity<br />
(6). FS is a clinically relevant parameter<br />
based on low-end, between-run precision.<br />
The guidelines define it as <strong>the</strong> Tg concentra-<br />
figure 1<br />
factors that influence Tg reference intervals<br />
schematic diagram of how <strong>the</strong> tg reference range is influenced by <strong>the</strong> degree of surgery: lobectomy<br />
(center panel) or near-total thyroidectomy (right panel), tsH status (on abscissa), and assay functional<br />
sensitivity (first- versus second-generation).<br />
Adapted from reference 6.<br />
tion that can be measured with 20% coefficient<br />
of variation determined from multiple<br />
measurements of a human serum pool containing<br />
a low Tg level made across a clinically-relevant<br />
time-span (6–12 months) and<br />
employing at least two calibrator lots and<br />
two reagent lots. The guidelines committee<br />
developed this definition to realistically represent<br />
<strong>the</strong> sensitivity of <strong>the</strong> test used in clinical<br />
practice, and to replace descriptive terms<br />
like “ultrasensitive” and “supersensitive”<br />
that manufacturers favor <strong>for</strong> marketing.<br />
First- Versus Second-Generation FS<br />
RIA methodology is only capable of firstgeneration<br />
FS ranging from 0.5–1.0 µg/L<br />
(3). Laboratorians hoped that replacing<br />
RIA with IMA methodology would improve<br />
FS by an order of magnitude, as was<br />
<strong>the</strong> case in <strong>the</strong> 1980s <strong>for</strong> TSH. Un<strong>for</strong>tunately,<br />
most current Tg IMA methods still only<br />
have first-generation FS, with a detection<br />
limit only marginally below <strong>the</strong> lower reference<br />
limit <strong>for</strong> control subjects with intact<br />
thyroid glands (Figure 1 and Table 1).<br />
First-generation assays are too insensitive<br />
clinically to use <strong>for</strong> basal Tg monitoring<br />
without TSH stimulation. As a result,<br />
it is customary to measure serum Tg after<br />
rhTSH stimulation, a maneuver analogous<br />
to <strong>the</strong> use of thyrotropin-releasing hormone<br />
stimulation to overcome <strong>the</strong> insensitivity<br />
of <strong>the</strong> TSH assays used be<strong>for</strong>e <strong>the</strong><br />
1990s (7). By consensus, a 72-hour rhTSHstimulated<br />
Tg above 2.0 µg/L is considered<br />
a risk factor <strong>for</strong> disease (7). In reality, this<br />
fixed rhTSH-Tg cutoff has a low positive<br />
predictive value <strong>for</strong> disease of about 50%.<br />
Fur<strong>the</strong>rmore, depending on <strong>the</strong> method<br />
used, a Tg value of 2.0 µg/L could be reported<br />
as being anywhere between 1.5 and<br />
3.2 µg/L using different methods (8).<br />
More recently, second-generation IMAs<br />
have become available with FS ranging<br />
table 1<br />
characteristics of current Tg assays<br />
from 0.05–0.1 µg/L (9). These more sensitive<br />
assays show that <strong>the</strong>re is a strong 10fold<br />
difference between basal and rhTSHstimulated<br />
Tg values, <strong>the</strong>reby obviating<br />
<strong>the</strong> need <strong>for</strong> expensive and inconvenient<br />
rhTSH-stimulated Tg testing <strong>for</strong> most patients<br />
(Figure 3) (8). Second generation<br />
IMAs also facilitate <strong>the</strong> monitoring of basal<br />
Tg trends that improve positive and negative<br />
predictive values <strong>for</strong> assessing risk <strong>for</strong><br />
disease, as compared with <strong>the</strong> rhTSH-Tg<br />
cutoff value of 2.0 µg/L (1,10,11).<br />
Notably, when TgAb is present, rhTSH<br />
stimulation offers no diagnostic benefit. This<br />
is because rhTSH-Tg responses are paradoxically<br />
blunted or absent in <strong>the</strong> presence<br />
of TgAb, possibly because of increased metabolic<br />
clearance of Tg-TgAb complexes (9).<br />
The Hook Effect<br />
A high-dose hook effect can occur when<br />
using IMA to measure exceedingly high Tg<br />
Assay # 1 2 3 4 5 6 7 8 9<br />
Assay Name Access Tg Tg-Plus TgIRMA Immulite 2000 Tg TgRIA Elecsys Tg Tg Wallac Delfia Tg<br />
Manufacturer<br />
Beckman Coulter,<br />
US<br />
BRAHMS<br />
diagnostica,<br />
Germany<br />
CISbio-Schering,<br />
Germany<br />
Siemens, US<br />
Genesis<br />
Diagnostics, UK<br />
University<br />
Sou<strong>the</strong>rn<br />
Cali<strong>for</strong>nia, US<br />
Roche,<br />
Germany<br />
Sanofi Pasteur,<br />
France<br />
Perkin-Elmer,<br />
Finland<br />
Assay Type<br />
non-competitive<br />
ICMA<br />
competitive<br />
IRMA<br />
competitive<br />
IRMA<br />
competitive<br />
ICMA<br />
non-competitive<br />
ELISA<br />
competitive<br />
RIA<br />
competitive<br />
IECMA<br />
competitive<br />
IRMA<br />
competitive<br />
IFMA<br />
Capture Antibody 4xMab pab (rabbit) 4xMab Mab pab (rabbit) pab (rabbit) Mab 4xMab Mab<br />
Tracer ap-Mab 125-iMab 125-iMab (sheep) ap-pab (rabbit) 125-itg Mab 125-iMab eu-Mab<br />
Sample Size µL 40 100 100 50 50 200 20 100 50<br />
1:1 CRM-457<br />
Standardization<br />
Functional<br />
yes<br />
no<br />
(factor of 2)<br />
yes yes no yes yes no no<br />
Sensitivity ng/mL<br />
(µg/L)*#<br />
Reference<br />
0.1 [5,1] 0.4* 0.7 0.9 na 0.5 1.0 na na<br />
Range ng/mL<br />
(µg/L)*<br />
3–32 [3] 2–34 [3] 2.1–43 [3] 1.6–60 2.0–50 3.0-40 1.4–78 1.5–50 1.7–35<br />
*expressed relative to 1:1 CrM-457 standardization; # defined by naCb guidelines (6); [ ] literature citation, no manufacturer data<br />
CliniCal laboratory news July 2011 11