Management of hand-foot syndrome in patients treated with ...
Management of hand-foot syndrome in patients treated with ...
Management of hand-foot syndrome in patients treated with ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
S34<br />
type <strong>of</strong> cytotoxic agent used. High rates <strong>of</strong> severe<br />
HFS have been reported <strong>with</strong> pegylated liposomal<br />
doxorubic<strong>in</strong> (D’Agost<strong>in</strong>o et al., 2003), and the<br />
condition appears to be more common and severe<br />
(<strong>in</strong>clud<strong>in</strong>g <strong>in</strong>fection and septicaemia) than <strong>in</strong><br />
<strong>patients</strong> receiv<strong>in</strong>g capecitab<strong>in</strong>e.<br />
Frequency <strong>of</strong> HFS <strong>with</strong> capecitab<strong>in</strong>e<br />
HFS (all grades) occurred <strong>in</strong> approximately 50% <strong>of</strong><br />
<strong>patients</strong> <strong>in</strong> early phase II studies <strong>of</strong> capecitab<strong>in</strong>e<br />
s<strong>in</strong>gle-agent therapy <strong>in</strong> metastatic breast cancer<br />
(MBC) (Blum et al., 1999) and metastatic colorectal<br />
cancer (MCRC) (Abushullaih et al., 2002; Van<br />
Cutsem et al., 2000), <strong>with</strong> 10% <strong>of</strong> <strong>patients</strong><br />
experienc<strong>in</strong>g severe (grade 3) HFS. A higher rate<br />
<strong>of</strong> severe HFS (52%, 12/23 cases) was reported <strong>in</strong> a<br />
Korean study <strong>of</strong> capecitab<strong>in</strong>e <strong>in</strong> comb<strong>in</strong>ation <strong>with</strong><br />
docetaxel (Park et al., 2003), although this may be<br />
related to the ability <strong>of</strong> docetaxel to cause both<br />
HFS and nail toxicity.<br />
Because HFS is subjectively reported, standardisation<br />
is a challenge and cross-study comparison is<br />
ARTICLE IN PRESS<br />
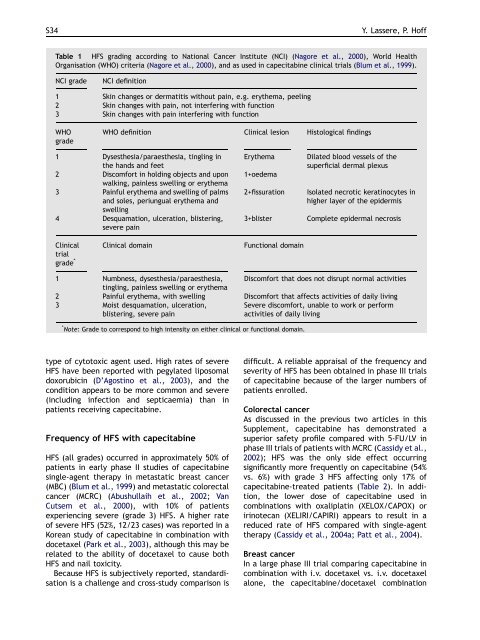
Table 1 HFS grad<strong>in</strong>g accord<strong>in</strong>g to National Cancer Institute (NCI) (Nagore et al., 2000), World Health<br />
Organisation (WHO) criteria (Nagore et al., 2000), and as used <strong>in</strong> capecitab<strong>in</strong>e cl<strong>in</strong>ical trials (Blum et al., 1999).<br />
NCI grade NCI def<strong>in</strong>ition<br />
1 Sk<strong>in</strong> changes or dermatitis <strong>with</strong>out pa<strong>in</strong>, e.g. erythema, peel<strong>in</strong>g<br />
2 Sk<strong>in</strong> changes <strong>with</strong> pa<strong>in</strong>, not <strong>in</strong>terfer<strong>in</strong>g <strong>with</strong> function<br />
3 Sk<strong>in</strong> changes <strong>with</strong> pa<strong>in</strong> <strong>in</strong>terfer<strong>in</strong>g <strong>with</strong> function<br />
WHO<br />
grade<br />
WHO def<strong>in</strong>ition Cl<strong>in</strong>ical lesion Histological f<strong>in</strong>d<strong>in</strong>gs<br />
1 Dysesthesia/paraesthesia, t<strong>in</strong>gl<strong>in</strong>g <strong>in</strong><br />
the <strong>hand</strong>s and feet<br />
2 Discomfort <strong>in</strong> hold<strong>in</strong>g objects and upon<br />
walk<strong>in</strong>g, pa<strong>in</strong>less swell<strong>in</strong>g or erythema<br />
3 Pa<strong>in</strong>ful erythema and swell<strong>in</strong>g <strong>of</strong> palms<br />
and soles, periungual erythema and<br />
swell<strong>in</strong>g<br />
4 Desquamation, ulceration, blister<strong>in</strong>g,<br />
severe pa<strong>in</strong><br />
Cl<strong>in</strong>ical<br />
trial<br />
grade *<br />
Cl<strong>in</strong>ical doma<strong>in</strong> Functional doma<strong>in</strong><br />
Erythema Dilated blood vessels <strong>of</strong> the<br />
superficial dermal plexus<br />
1+oedema<br />
2+fissuration Isolated necrotic kerat<strong>in</strong>ocytes <strong>in</strong><br />
higher layer <strong>of</strong> the epidermis<br />
3+blister Complete epidermal necrosis<br />
1 Numbness, dysesthesia/paraesthesia,<br />
t<strong>in</strong>gl<strong>in</strong>g, pa<strong>in</strong>less swell<strong>in</strong>g or erythema<br />
Discomfort that does not disrupt normal activities<br />
2 Pa<strong>in</strong>ful erythema, <strong>with</strong> swell<strong>in</strong>g Discomfort that affects activities <strong>of</strong> daily liv<strong>in</strong>g<br />
3 Moist desquamation, ulceration,<br />
Severe discomfort, unable to work or perform<br />
blister<strong>in</strong>g, severe pa<strong>in</strong><br />
activities <strong>of</strong> daily liv<strong>in</strong>g<br />
* Note: Grade to correspond to high <strong>in</strong>tensity on either cl<strong>in</strong>ical or functional doma<strong>in</strong>.<br />
Y. Lassere, P. H<strong>of</strong>f<br />
difficult. A reliable appraisal <strong>of</strong> the frequency and<br />
severity <strong>of</strong> HFS has been obta<strong>in</strong>ed <strong>in</strong> phase III trials<br />
<strong>of</strong> capecitab<strong>in</strong>e because <strong>of</strong> the larger numbers <strong>of</strong><br />
<strong>patients</strong> enrolled.<br />
Colorectal cancer<br />
As discussed <strong>in</strong> the previous two articles <strong>in</strong> this<br />
Supplement, capecitab<strong>in</strong>e has demonstrated a<br />
superior safety pr<strong>of</strong>ile compared <strong>with</strong> 5-FU/LV <strong>in</strong><br />
phase III trials <strong>of</strong> <strong>patients</strong> <strong>with</strong> MCRC (Cassidy et al.,<br />
2002); HFS was the only side effect occurr<strong>in</strong>g<br />
significantly more frequently on capecitab<strong>in</strong>e (54%<br />
vs. 6%) <strong>with</strong> grade 3 HFS affect<strong>in</strong>g only 17% <strong>of</strong><br />
capecitab<strong>in</strong>e-<strong>treated</strong> <strong>patients</strong> (Table 2). In addition,<br />
the lower dose <strong>of</strong> capecitab<strong>in</strong>e used <strong>in</strong><br />
comb<strong>in</strong>ations <strong>with</strong> oxaliplat<strong>in</strong> (XELOX/CAPOX) or<br />
ir<strong>in</strong>otecan (XELIRI/CAPIRI) appears to result <strong>in</strong> a<br />
reduced rate <strong>of</strong> HFS compared <strong>with</strong> s<strong>in</strong>gle-agent<br />
therapy (Cassidy et al., 2004a; Patt et al., 2004).<br />
Breast cancer<br />
In a large phase III trial compar<strong>in</strong>g capecitab<strong>in</strong>e <strong>in</strong><br />
comb<strong>in</strong>ation <strong>with</strong> i.v. docetaxel vs. i.v. docetaxel<br />
alone, the capecitab<strong>in</strong>e/docetaxel comb<strong>in</strong>ation