Management of hand-foot syndrome in patients treated with ...
Management of hand-foot syndrome in patients treated with ...
Management of hand-foot syndrome in patients treated with ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
S36<br />
cycle. An example would be <strong>in</strong>creas<strong>in</strong>g the <strong>in</strong>terval<br />
between cycles to 2 weeks or chang<strong>in</strong>g the cycle<br />
length (e.g. 10 days on, 11 days <strong>of</strong>f).<br />
ARTICLE IN PRESS<br />
Table 3 Capecitab<strong>in</strong>e dose-modification scheme for all adverse events.<br />
NCI-CTC toxicity<br />
grade<br />
Ma<strong>in</strong>ta<strong>in</strong><br />
<strong>in</strong>terruption<br />
Appearance <strong>of</strong><br />
toxicity<br />
Adjustment dur<strong>in</strong>g therapy Adjustment for<br />
next cycle<br />
(relative to<br />
<strong>in</strong>itial dose)<br />
2 1st Interrupt until resolved to grade 0/1 100%<br />
2nd Interrupt until resolved to grade 0/1 75%<br />
3rd Interrupt until resolved to grade 0/1 50%<br />
4th Discont<strong>in</strong>ue drug permanently<br />
3 1st Interrupt until resolved to grade 0/1 75%<br />
2nd Interrupt until resolved to grade 0/1 50%<br />
3rd Discont<strong>in</strong>ue drug permanently<br />
4 a<br />
1st Discont<strong>in</strong>ue drug permanently or <strong>in</strong>terrupt until<br />
resolved to grade 0/1 b<br />
50%<br />
NCI-CTC, National Cancer Institute Common Toxicity Criteria.<br />
a<br />
Not applicable to HFS.<br />
b<br />
At discretion <strong>of</strong> the cl<strong>in</strong>ician.<br />
NO<br />
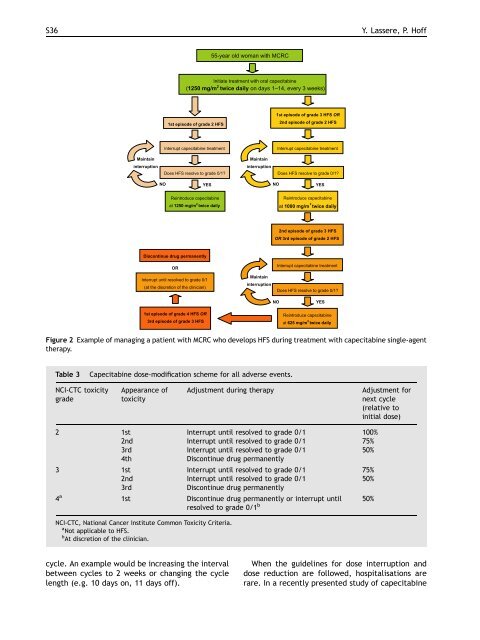
55-year old woman <strong>with</strong> MCRC<br />
Initiate treatment <strong>with</strong> oral capecitab<strong>in</strong>e<br />
(1250 mg/m 2 twice daily on days 1–14, every 3 weeks)<br />
1st episode <strong>of</strong> grade 2 HFS<br />
Interrupt capecitab<strong>in</strong>e treatment<br />
Does HFS resolve to grade 0/1?<br />
YES<br />
Re<strong>in</strong>troduce capecitab<strong>in</strong>e<br />
at 1250 mg/m twice daily<br />
Discont<strong>in</strong>ue drug permanently<br />
OR<br />
Interrupt until resolved to grade 0/1<br />
(at the discretion <strong>of</strong> the cl<strong>in</strong>ician)<br />
1st episode <strong>of</strong> grade 4 HFS OR<br />
3rd episode <strong>of</strong> grade 3 HFS<br />
Ma<strong>in</strong>ta<strong>in</strong><br />
<strong>in</strong>terruption<br />
Ma<strong>in</strong>ta<strong>in</strong><br />
<strong>in</strong>terruption<br />
1st episode <strong>of</strong> grade 3 HFS OR<br />
2nd episode <strong>of</strong> grade 2 HFS<br />
NO<br />
Interrupt capecitab<strong>in</strong>e treatment<br />
Does HFS resolve to grade 0/1?<br />
YES<br />
Re<strong>in</strong>troduce capecitab<strong>in</strong>e<br />
at 1000 mg/m twice daily<br />
2nd episode <strong>of</strong> grade 3 HFS<br />
OR 3rd episode <strong>of</strong> grade 2 HFS<br />
NO<br />
Interrupt capecitab<strong>in</strong>e treatment<br />
Does HFS resolve to grade 0/1?<br />
YES<br />
Re<strong>in</strong>troduce capecitab<strong>in</strong>e<br />
at 625 mg/m twice daily<br />
Y. Lassere, P. H<strong>of</strong>f<br />
Figure 2 Example <strong>of</strong> manag<strong>in</strong>g a patient <strong>with</strong> MCRC who develops HFS dur<strong>in</strong>g treatment <strong>with</strong> capecitab<strong>in</strong>e s<strong>in</strong>gle-agent<br />
therapy.<br />
When the guidel<strong>in</strong>es for dose <strong>in</strong>terruption and<br />
dose reduction are followed, hospitalisations are<br />
rare. In a recently presented study <strong>of</strong> capecitab<strong>in</strong>e