PDF-format - Miljøstyrelsen
PDF-format - Miljøstyrelsen
PDF-format - Miljøstyrelsen
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
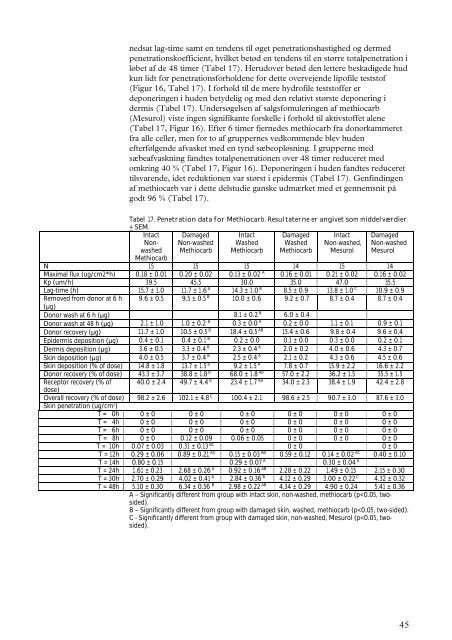
nedsat lag-time samt en tendens til øget penetrationshastighed og dermedpenetrationskoefficient, hvilket betød en tendens til en større totalpenetration iløbet af de 48 timer (Tabel 17). Herudover betød den lettere beskadigede hudkun lidt for penetrationsforholdene for dette overvejende lipofile teststof(Figur 16, Tabel 17). I forhold til de mere hydrofile teststoffer erdeponeringen i huden betydelig og med den relativt største deponering idermis (Tabel 17). Undersøgelsen af salgsfomuleringen af methiocarb(Mesurol) viste ingen signifikante forskelle i forhold til aktivstoffet alene(Tabel 17, Figur 16). Efter 6 timer fjernedes methiocarb fra donorkammeretfra alle celler, men for to af gruppernes vedkommende blev hudenefterfølgende afvasket med en tynd sæbeopløsning. I grupperne medsæbeafvaskning fandtes totalpenetrationen over 48 timer reduceret medomkring 40 % (Tabel 17, Figur 16). Deponeringen i huden fandtes reducerettilsvarende, idet reduktionen var størst i epidermis (Tabel 17). Genfindingenaf methiocarb var i dette delstudie ganske udmærket med et gennemsnit pågodt 96 % (Tabel 17).Tabel 17. Penetration data for Methiocarb. Resultaterne er angivet som middelværdier+ SEM.IntactNonwashedDamagedNon-washedMethiocarbIntactWashedMethiocarbDamagedWashedMethiocarbIntactNon-washed,MesurolDamagedNon-washedMesurolMethiocarbN 15 15 15 14 15 14Maximal flux (ug/cm2*h) 0.18 ± 0.01 0.20 ± 0.02 0.13 ± 0.02 A 0.16 ± 0.01 0.21 ± 0.02 0.16 ± 0.02Kp (um/h) 39.5 45.5 30.0 35.0 47.0 35.5Lag-time (h) 15.7 ± 1.0 11.7 ± 1.6 A 14.3 ± 1.0 B 8.5 ± 0.9 13.8 ± 1.0 C 10.9 ± 0.9Removed from donor at 6 h 9.6 ± 0.5 9.5 ± 0.5 B 10.0 ± 0.6 9.2 ± 0.7 8.7 ± 0.4 8.7 ± 0.4(µg)Donor wash at 6 h (µg) 8.1 ± 0.2 B 6.0 ± 0.4Donor wash at 48 h (µg) 2.1 ± 1.0 1.0 ± 0.2 B 0.3 ± 0.0 A 0.2 ± 0.0 1.1 ± 0.1 0.9 ± 0.1Donor recovery (µg) 11.7 ± 1.0 10.5 ± 0.5 B 18.4 ± 0.5 AB 15.4 ± 0.6 9.8 ± 0.4 9.6 ± 0.4Epidermis deposition (µg) 0.4 ± 0.1 0.4 ± 0.1 B 0.2 ± 0.0 0.1 ± 0.0 0.3 ± 0.0 0.2 ± 0.1Dermis deposition (µg) 3.6 ± 0.5 3.3 ± 0.4 B 2.3 ± 0.4 A 2.0 ± 0.2 4.0 ± 0.6 4.3 ± 0.7Skin deposition (µg) 4.0 ± 0.5 3.7 ± 0.4 B 2.5 ± 0.4 A 2.1 ± 0.2 4.3 ± 0.6 4.5 ± 0.6Skin deposition (% of dose) 14.8 ± 1.8 13.7 ± 1.5 B 9.2 ± 1.5 A 7.8 ± 0.7 15.9 ± 2.2 16.6 ± 2.2Donor recovery (% of dose) 43.3 ± 3.7 38.8 ± 1.8 B 68.0 ± 1.8 AB 57.0 ± 2.2 36.2 ± 1.5 35.5 ± 1.5Receptor recovery (% of 40.0 ± 2.4 49.7 ± 4.4 B 23.4 ± 1.7 AB 34.0 ± 2.3 38.4 ± 1.9 42.4 ± 2.8dose)Overall recovery (% of dose) 98.2 ± 2.6 102.1 ± 4.8 C 100.4 ± 2.1 98.6 ± 2.5 90.7 ± 3.0 87.6 ± 3.0Skin penetration (ug/cm 2 )T = 0h 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0T = 4h 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0T = 6h 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0T = 8h 0 ± 0 0.12 ± 0.09 0.06 ± 0.05 0 ± 0 0 ± 0 0 ± 0T = 10h 0.07 ± 0.03 0.31 ± 0.13 BC 0 ± 0 0 ± 0T = 12h 0.29 ± 0.06 0.89 ± 0.21 AC 0.15 ± 0.03 AB 0.59 ± 0.12 0.14 ± 0.02 AC 0.40 ± 0.10T = 14h 0.80 ± 0.15 0.29 ± 0.07 A 0.30 ± 0.04 AT = 24h 1.61 ± 0.23 2.68 ± 0.26 A 0.92 ± 0.16 AB 2.28 ± 0.22 1.49 ± 0.15 2.15 ± 0.30T = 30h 2.70 ± 0.29 4.02 ± 0.41 A 2.84 ± 0.36 B 4.12 ± 0.29 3.00 ± 0.22 C 4.32 ± 0.32T = 48h 5.10 ± 0.30 6.34 ± 0.56 B 2.98 ± 0.22 AB 4.34 ± 0.29 4.90 ± 0.24 5.41 ± 0.36A – Significantly different from group with intact skin, non-washed, methiocarb (p