7th Annual AMIA Invitational Health Policy Meeting December 12-13 ...
7th Annual AMIA Invitational Health Policy Meeting December 12-13 ...
7th Annual AMIA Invitational Health Policy Meeting December 12-13 ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
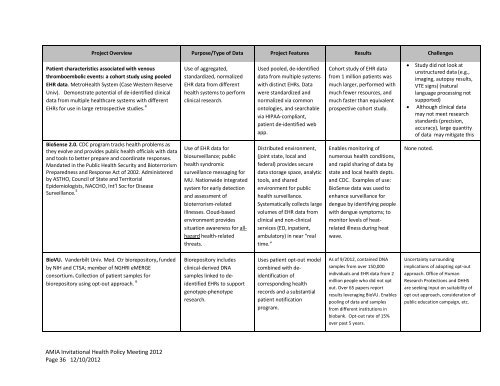
Project Overview Purpose/Type of Data Project Features Results Challenges<br />
Patient characteristics associated with venous<br />
thromboembolic events: a cohort study using pooled<br />
EHR data. Metro<strong>Health</strong> System (Case Western Reserve<br />
Univ). Demonstrate potential of de-identified clinical<br />
data from multiple healthcare systems with different<br />
EHRs for use in large retrospective studies. 4<br />
BioSense 2.0. CDC program tracks health problems as<br />
they evolve and provides public health officials with data<br />
and tools to better prepare and coordinate responses.<br />
Mandated in the Public <strong>Health</strong> Security and Bioterrorism<br />
Preparedness and Response Act of 2002. Administered<br />
by ASTHO, Council of State and Territorial<br />
Epidemiologists, NACCHO, Int’l Soc for Disease<br />
Surveillance. 5<br />
BioVU. Vanderbilt Univ. Med. Ctr biorepository, funded<br />
by NIH and CTSA; member of NGHRI eMERGE<br />
consortium. Collection of patient samples for<br />
biorepository using opt-out approach. 6<br />
<strong>AMIA</strong> <strong>Invitational</strong> <strong>Health</strong> <strong>Policy</strong> <strong>Meeting</strong> 20<strong>12</strong><br />
Page 36 <strong>12</strong>/10/20<strong>12</strong><br />
Use of aggregated,<br />
standardized, normalized<br />
EHR data from different<br />
health systems to perform<br />
clinical research.<br />
Use of EHR data for<br />
biosurveillance; public<br />
health syndromic<br />
surveillance messaging for<br />
MU. Nationwide integrated<br />
system for early detection<br />
and assessment of<br />
bioterrorism-related<br />
illnesses. Cloud-based<br />
environment provides<br />
situation awareness for allhazard<br />
health-related<br />
threats.<br />
Biorepository includes<br />
clinical-derived DNA<br />
samples linked to deidentified<br />
EHRs to support<br />
genotype-phenotype<br />
research.<br />
Used pooled, de-identified<br />
data from multiple systems<br />
with distinct EHRs. Data<br />
were standardized and<br />
normalized via common<br />
ontologies, and searchable<br />
via HIPAA-compliant,<br />
patient de-identified web<br />
app.<br />
Distributed environment,<br />
(joint state, local and<br />
federal) provides secure<br />
data storage space, analytic<br />
tools, and shared<br />
environment for public<br />
health surveillance.<br />
Systematically collects large<br />
volumes of EHR data from<br />
clinical and non-clinical<br />
services (ED, inpatient,<br />
ambulatory) in near “real<br />
time.”<br />
Uses patient opt-out model<br />
combined with deidentification<br />
of<br />
corresponding health<br />
records and a substantial<br />
patient notification<br />
program.<br />
Cohort study of EHR data<br />
from 1 million patients was<br />
much larger, performed with<br />
much fewer resources, and<br />
much faster than equivalent<br />
prospective cohort study.<br />
Enables monitoring of<br />
numerous health conditions,<br />
and rapid sharing of data by<br />
state and local health depts.<br />
and CDC. Examples of use:<br />
BioSense data was used to<br />
enhance surveillance for<br />
dengue by identifying people<br />
with dengue symptoms; to<br />
monitor levels of heatrelated<br />
illness during heat<br />
wave.<br />
As of 9/20<strong>12</strong>, contained DNA<br />
samples from over 150,000<br />
individuals and EHR data from 2<br />
million people who did not opt<br />
out. Over 65 papers report<br />
results leveraging BioVU. Enables<br />
pooling of data and samples<br />
from different institutions in<br />
biobank. Opt-out rate of 15%<br />
over past 5 years.<br />
� Study did not look at<br />
unstructured data (e.g.,<br />
imaging, autopsy results,<br />
VTE signs) (natural<br />
language processing not<br />
supported)<br />
� Although clinical data<br />
may not meet research<br />
standards (precision,<br />
accuracy), large quantity<br />
of data may mitigate this<br />
None noted.<br />
Uncertainty surrounding<br />
implications of adopting opt-out<br />
approach. Office of Human<br />
Research Protections and DHHS<br />
are seeking input on suitability of<br />
opt out approach, consideration of<br />
public education campaign, etc.