7th Annual AMIA Invitational Health Policy Meeting December 12-13 ...
7th Annual AMIA Invitational Health Policy Meeting December 12-13 ...
7th Annual AMIA Invitational Health Policy Meeting December 12-13 ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
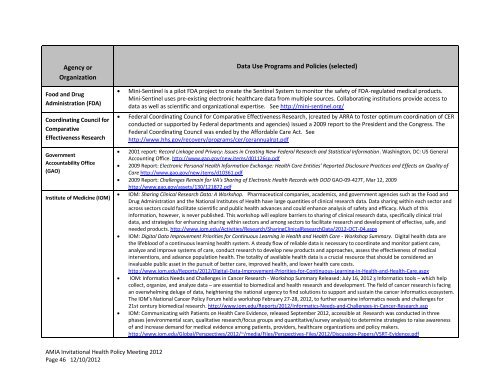
Agency or<br />
Organization<br />
Food and Drug<br />
Administration (FDA)<br />
Coordinating Council for<br />
Comparative<br />
Effectiveness Research<br />
Government<br />
Accountability Office<br />
(GAO)<br />
Institute of Medicine (IOM)<br />
<strong>AMIA</strong> <strong>Invitational</strong> <strong>Health</strong> <strong>Policy</strong> <strong>Meeting</strong> 20<strong>12</strong><br />
Page 46 <strong>12</strong>/10/20<strong>12</strong><br />
Data Use Programs and Policies (selected)<br />
� Mini-Sentinel is a pilot FDA project to create the Sentinel System to monitor the safety of FDA-regulated medical products.<br />
Mini-Sentinel uses pre-existing electronic healthcare data from multiple sources. Collaborating institutions provide access to<br />
data as well as scientific and organizational expertise. See http://mini-sentinel.org/<br />
� Federal Coordinating Council for Comparative Effectiveness Research, (created by ARRA to foster optimum coordination of CER<br />
conducted or supported by Federal departments and agencies) issued a 2009 report to the President and the Congress. The<br />
Federal Coordinating Council was ended by the Affordable Care Act. See<br />
http://www.hhs.gov/recovery/programs/cer/cerannualrpt.pdf<br />
� 2001 report: Record Linkage and Privacy: Issues in Creating New Federal Research and Statistical Information. Washington, DC: US General<br />
Accounting Office. http://www.gao.gov/new.items/d01<strong>12</strong>6sp.pdf<br />
� 2009 Report: Electronic Personal <strong>Health</strong> Information Exchange: <strong>Health</strong> Care Entities’ Reported Disclosure Practices and Effects on Quality of<br />
Care http://www.gao.gov/new.items/d10361.pdf<br />
� 2009 Report: Challenges Remain for VA's Sharing of Electronic <strong>Health</strong> Records with DOD GAO-09-427T, Mar <strong>12</strong>, 2009<br />
http://www.gao.gov/assets/<strong>13</strong>0/<strong>12</strong>1872.pdf<br />
� IOM: Sharing Clinical Research Data: A Workshop. Pharmaceutical companies, academics, and government agencies such as the Food and<br />
Drug Administration and the National Institutes of <strong>Health</strong> have large quantities of clinical research data. Data sharing within each sector and<br />
across sectors could facilitate scientific and public health advances and could enhance analysis of safety and efficacy. Much of this<br />
information, however, is never published. This workshop will explore barriers to sharing of clinical research data, specifically clinical trial<br />
data, and strategies for enhancing sharing within sectors and among sectors to facilitate research and development of effective, safe, and<br />
needed products. http://www.iom.edu/Activities/Research/SharingClinicalResearchData/20<strong>12</strong>-OCT-04.aspx<br />
� IOM: Digital Data Improvement Priorities for Continuous Learning in <strong>Health</strong> and <strong>Health</strong> Care - Workshop Summary. Digital health data are<br />
the lifeblood of a continuous learning health system. A steady flow of reliable data is necessary to coordinate and monitor patient care,<br />
analyze and improve systems of care, conduct research to develop new products and approaches, assess the effectiveness of medical<br />
interventions, and advance population health. The totality of available health data is a crucial resource that should be considered an<br />
invaluable public asset in the pursuit of better care, improved health, and lower health care costs.<br />
http://www.iom.edu/Reports/20<strong>12</strong>/Digital-Data-Improvement-Priorities-for-Continuous-Learning-in-<strong>Health</strong>-and-<strong>Health</strong>-Care.aspx<br />
� IOM: Informatics Needs and Challenges in Cancer Research - Workshop Summary Released: July 16, 20<strong>12</strong> x Informatics tools – which help<br />
collect, organize, and analyze data – are essential to biomedical and health research and development. The field of cancer research is facing<br />
an overwhelming deluge of data, heightening the national urgency to find solutions to support and sustain the cancer informatics ecosystem.<br />
The IOM’s National Cancer <strong>Policy</strong> Forum held a workshop February 27-28, 20<strong>12</strong>, to further examine informatics needs and challenges for<br />
21st century biomedical research. http://www.iom.edu/Reports/20<strong>12</strong>/Informatics-Needs-and-Challenges-in-Cancer-Research.asp<br />
� IOM: Communicating with Patients on <strong>Health</strong> Care Evidence, released September 20<strong>12</strong>, accessible at Research was conducted in three<br />
phases (environmental scan, qualitative research/focus groups and quantitative/survey analysis) to determine strategies to raise awareness<br />
of and increase demand for medical evidence among patients, providers, healthcare organizations and policy makers.<br />
http://www.iom.edu/Global/Perspectives/20<strong>12</strong>/~/media/Files/Perspectives-Files/20<strong>12</strong>/Discussion-Papers/VSRT-Evidence.pdf