The Röntgen Radiation and its application in studies of ... - Mansic
The Röntgen Radiation and its application in studies of ... - Mansic
The Röntgen Radiation and its application in studies of ... - Mansic
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Due to the fact that Kβ <strong>and</strong> Kα2 are diffracted on the same crystallographic plane,<br />
they cause appearance <strong>of</strong> additional peaks <strong>in</strong> XRD pattern. Such <strong>in</strong>convenience<br />
can be easy elim<strong>in</strong>ated by add<strong>in</strong>g filters.<br />
4. Interaction with matter<br />
<strong>The</strong> absorption <strong>of</strong> the X rays<br />
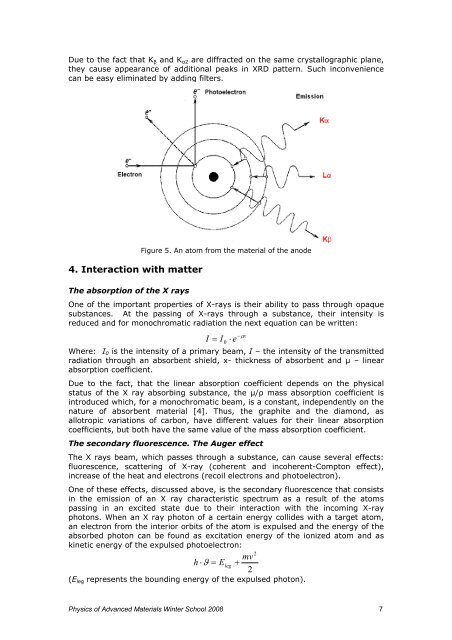
Figure 5. An atom from the material <strong>of</strong> the anode<br />
One <strong>of</strong> the important properties <strong>of</strong> X-rays is their ability to pass through opaque<br />
substances. At the pass<strong>in</strong>g <strong>of</strong> X-rays through a substance, their <strong>in</strong>tensity is<br />
reduced <strong>and</strong> for monochromatic radiation the next equation can be written:<br />
I = I 0 ⋅ e<br />
Where: I0 is the <strong>in</strong>tensity <strong>of</strong> a primary beam, I – the <strong>in</strong>tensity <strong>of</strong> the transmitted<br />
radiation through an absorbent shield, x- thickness <strong>of</strong> absorbent <strong>and</strong> µ – l<strong>in</strong>ear<br />
absorption coefficient.<br />
Due to the fact, that the l<strong>in</strong>ear absorption coefficient depends on the physical<br />
status <strong>of</strong> the X ray absorb<strong>in</strong>g substance, the µ/ρ mass absorption coefficient is<br />
<strong>in</strong>troduced which, for a monochromatic beam, is a constant, <strong>in</strong>dependently on the<br />
nature <strong>of</strong> absorbent material [4]. Thus, the graphite <strong>and</strong> the diamond, as<br />
allotropic variations <strong>of</strong> carbon, have different values for their l<strong>in</strong>ear absorption<br />
coefficients, but both have the same value <strong>of</strong> the mass absorption coefficient.<br />
Physics <strong>of</strong> Advanced Materials W<strong>in</strong>ter School 2008 7<br />
−µ<br />
x<br />
<strong>The</strong> secondary fluorescence. <strong>The</strong> Auger effect<br />
<strong>The</strong> X rays beam, which passes through a substance, can cause several effects:<br />
fluorescence, scatter<strong>in</strong>g <strong>of</strong> X-ray (coherent <strong>and</strong> <strong>in</strong>coherent-Compton effect),<br />
<strong>in</strong>crease <strong>of</strong> the heat <strong>and</strong> electrons (recoil electrons <strong>and</strong> photoelectron).<br />
One <strong>of</strong> these effects, discussed above, is the secondary fluorescence that consists<br />
<strong>in</strong> the emission <strong>of</strong> an X ray characteristic spectrum as a result <strong>of</strong> the atoms<br />
pass<strong>in</strong>g <strong>in</strong> an excited state due to their <strong>in</strong>teraction with the <strong>in</strong>com<strong>in</strong>g X-ray<br />
photons. When an X ray photon <strong>of</strong> a certa<strong>in</strong> energy collides with a target atom,<br />
an electron from the <strong>in</strong>terior orb<strong>its</strong> <strong>of</strong> the atom is expulsed <strong>and</strong> the energy <strong>of</strong> the<br />
absorbed photon can be found as excitation energy <strong>of</strong> the ionized atom <strong>and</strong> as<br />
k<strong>in</strong>etic energy <strong>of</strong> the expulsed photoelectron:<br />
mv<br />
h Eleg<br />
2<br />
+ = ⋅ϑ<br />
(Eleg represents the bound<strong>in</strong>g energy <strong>of</strong> the expulsed photon).<br />
2