MEDICINAL CHEMISTRY
MEDICINAL CHEMISTRY
MEDICINAL CHEMISTRY
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
conduction velocity, and decrease spontaneous diastolic depolarization in pacemaker<br />
cells. The decrease in diastolic depolarization tends to suppress ectopic foci activity.<br />
Prolongation of the refractory period tends to abolish reentry arrhythmias. This class is<br />
further subclassified into class IA, IB, and IC based on the primary pharmacologic effect.<br />
Class IA Antiarrhythmic Drugs<br />
H 3CO<br />
6'<br />
H<br />
HO<br />
C N<br />
9<br />
H<br />
8<br />
N<br />
H<br />
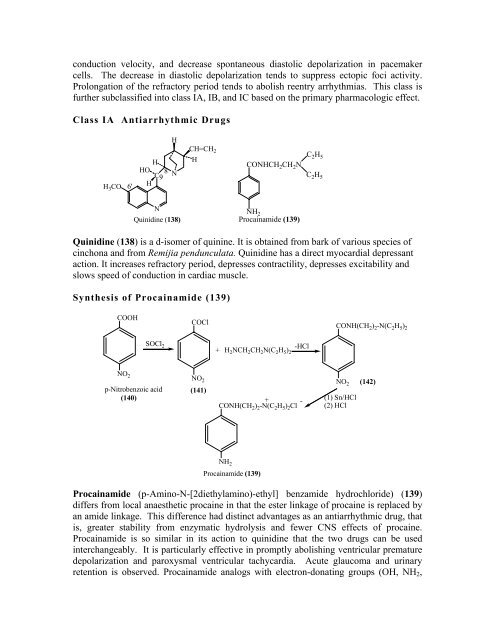
Quinidine (138)<br />
CH=CH 2<br />
H<br />
CONHCH 2CH 2N<br />
NH 2<br />
Procainamide (139)<br />
Quinidine (138) is a d-isomer of quinine. It is obtained from bark of various species of<br />
cinchona and from Remijia pendunculata. Quinidine has a direct myocardial depressant<br />
action. It increases refractory period, depresses contractility, depresses excitability and<br />
slows speed of conduction in cardiac muscle.<br />
Synthesis of Procainamide (139)<br />
COOH<br />
NO 2<br />
SOCl 2<br />
p-Nitrobenzoic acid<br />
(140)<br />
COCl<br />
NO 2<br />
(141)<br />
-HCl<br />
+ H2NCH2CH2N(C2H5) 2<br />
+ -<br />
CONH(CH2) 2-N(C2H5) 2Cl<br />
NH 2<br />
Procainamide (139)<br />
C 2H 5<br />
C 2H 5<br />
CONH(CH 2) 2-N(C 2H 5) 2<br />
NO 2<br />
(1) Sn/HCl<br />
(2) HCl<br />
Procainamide (p-Amino-N-[2diethylamino)-ethyl] benzamide hydrochloride) (139)<br />
differs from local anaesthetic procaine in that the ester linkage of procaine is replaced by<br />
an amide linkage. This difference had distinct advantages as an antiarrhythmic drug, that<br />
is, greater stability from enzymatic hydrolysis and fewer CNS effects of procaine.<br />
Procainamide is so similar in its action to quinidine that the two drugs can be used<br />
interchangeably. It is particularly effective in promptly abolishing ventricular premature<br />
depolarization and paroxysmal ventricular tachycardia. Acute glaucoma and urinary<br />
retention is observed. Procainamide analogs with electron-donating groups (OH, NH2,<br />
(142)