MEDICINAL CHEMISTRY
MEDICINAL CHEMISTRY
MEDICINAL CHEMISTRY
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Tocainide Hydrochloride (±)-2-Amino-N-(2,6-dimethyl-phenyl) propionamide<br />
hydrochloride (157) is another lidocaine congener, similar to mexiletine its electiophysiologic<br />
properties and antiarrhythmic action; A low incidence of bone marrow<br />
depression has caused this drug to be used less frequently than mexiletine.<br />
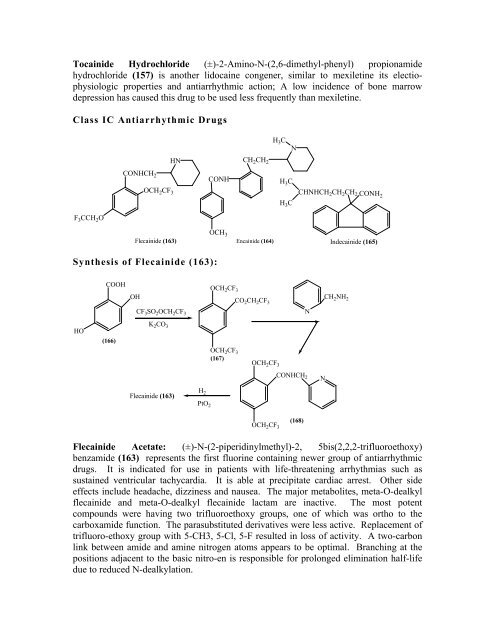
Class IC Antiarrhythmic Drugs<br />
F 3CCH 2O<br />
CONHCH 2<br />
OCH 2CF 3<br />
HN<br />
CONH<br />
CH 2CH 2<br />
H 3C<br />
H 3C<br />
H 3C<br />
N<br />
CHNHCH2CH2CH2 CONH2 OCH3 Flecainide (163) Encainide (164)<br />
Indecainide (165)<br />
Synthesis of Flecainide (163):<br />
HO<br />
COOH<br />
(166)<br />
OH<br />
CF 3SO 2OCH 2CF 3<br />
K 2CO 3<br />
Flecainide (163)<br />
H 2<br />
PtO 2<br />
OCH 2CF 3<br />
OCH2CF3 (167)<br />
CO 2CH 2CF 3<br />
OCH 2CF 3<br />
OCH 2CF 3<br />
N<br />
CONHCH 2<br />
(168)<br />
N<br />
CH 2NH 2<br />
Flecainide Acetate: (±)-N-(2-piperidinylmethyl)-2, 5bis(2,2,2-trifluoroethoxy)<br />
benzamide (163) represents the first fluorine containing newer group of antiarrhythmic<br />
drugs. It is indicated for use in patients with life-threatening arrhythmias such as<br />
sustained ventricular tachycardia. It is able at precipitate cardiac arrest. Other side<br />
effects include headache, dizziness and nausea. The major metabolites, meta-O-dealkyl<br />
flecainide and meta-O-dealkyl flecainide lactam are inactive. The most potent<br />
compounds were having two trifluoroethoxy groups, one of which was ortho to the<br />
carboxamide function. The parasubstituted derivatives were less active. Replacement of<br />
trifluoro-ethoxy group with 5-CH3, 5-Cl, 5-F resulted in loss of activity. A two-carbon<br />
link between amide and amine nitrogen atoms appears to be optimal. Branching at the<br />
positions adjacent to the basic nitro-en is responsible for prolonged elimination half-life<br />
due to reduced N-dealkylation.