HPLC Analysis of Biomolecules Technical Guide - Interscience
HPLC Analysis of Biomolecules Technical Guide - Interscience
HPLC Analysis of Biomolecules Technical Guide - Interscience
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
24<br />
<strong>HPLC</strong> <strong>Analysis</strong> <strong>of</strong> <strong>Biomolecules</strong><br />
The anion exchange process may be represented by the<br />
equilibrium [3]:<br />
[3] ~NH + C - + X - = ~NH + X - + C -<br />
where C - is the counter ion to the fixed ion exchange site, ~NH + ,<br />
present in the eluent. Analyte X - can then displace C - to give the ion<br />
pair NH + X - . For effective chromatography, this displacement reaction<br />
must be at equilibrium. Retention is therefore controlled by the<br />
equilibrium [3] for which the equilibrium constant K IE is given by<br />
equation [4]:<br />
[4]<br />
K IE = [C- ] [~NH + X - ]<br />
[X - ] [~NH + C - ]<br />
The capacity factor, k, <strong>of</strong> an analyte (X - ) is a more useful<br />
chromatographic parameter and is proportional to the distribution<br />
coefficient, D IE . Its relationship to the equilibrium constant, K IE , is<br />
given by equation [5]:<br />
[5]<br />
K ∝ D IE = [~NH+ X - ] = KIE • [~NH+ C - ]<br />
[X - ] [C - ]<br />
Since the concentration <strong>of</strong> the ion exchange sites, [~NH + X - ], is<br />
constant and fixed by the rigid structure <strong>of</strong> the matrix, k is inversely<br />
proportional to the concentration <strong>of</strong> the counter ion (ionic strength)<br />
in the eluent: Desorption is then brought about by increasing the<br />
salt concentration as shown by equation [6].<br />
[6] K ∝ [C - ] -1<br />
Particles for Ion Exchange<br />
Both polymer- and silica-based particles are available for ion<br />
exchange separations <strong>of</strong> biomolecules. Polymer-based particles<br />
have the advantage <strong>of</strong> extended pH range (chemical stability), while<br />
silica-based particles have higher efficiency and better mechanical<br />
strength. However, there are hybrid particles that <strong>of</strong>fer the benefits<br />
<strong>of</strong> both. An example is the BioBasic ion exchangers that comprise<br />
high purity silica with a polymeric coating. The polymeric coating<br />
extends the usable pH range, and also shields proteins from adsorbing<br />
to the silica surface.<br />
Particle Size, Pore Size, and Column Length<br />
The effects <strong>of</strong> these variables in ion exchange are largely the same<br />
as for RP-<strong>HPLC</strong>, discussed previously. Efficiency (resolution) increases<br />
with decreasing particle size and increasing column length, but at<br />
the expense <strong>of</strong> higher back pressure for smaller particles and longer<br />
columns. Longer columns also increase analysis time. Pore size<br />
should be larger, 300Å or greater, for proteins and large peptides<br />
to ensure access to the interior <strong>of</strong> the pores where the majority <strong>of</strong><br />
the surface area resides.<br />
Flow Rate<br />
High column efficiency (number <strong>of</strong> theoretical plates) is an important<br />
factor to consider when maximizing the resolution <strong>of</strong> a separation.<br />
In ion exchange chromatography the efficiency (sharpness) <strong>of</strong> a peak<br />
is governed not only by how well the column is packed but also by<br />
the mass transfer characteristics <strong>of</strong> the ion exchange mechanism<br />
itself. The term mass transfer used here refers to the efficient<br />
migration <strong>of</strong> the analyte from the mobile phase on to the stationary<br />
phase ion exchange site and then back into the mobile phase from<br />
the ion exchange site. If this process is not very rapid, broad peaks<br />
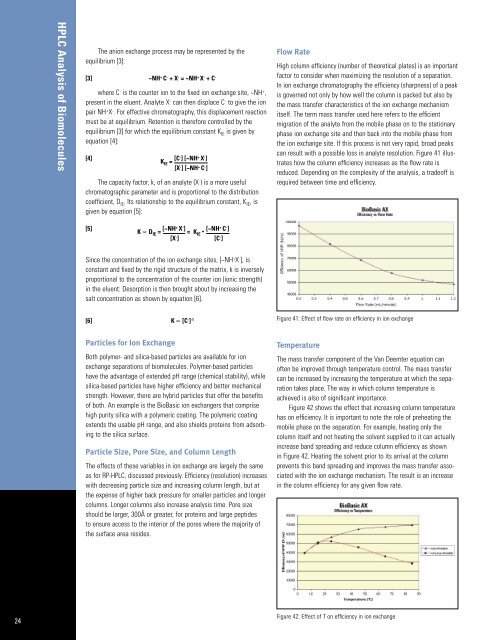
can result with a possible loss in analyte resolution. Figure 41 illustrates<br />
how the column efficiency increases as the flow rate is<br />
reduced. Depending on the complexity <strong>of</strong> the analysis, a trade<strong>of</strong>f is<br />
required between time and efficiency.<br />
Figure 41: Effect <strong>of</strong> flow rate on efficiency in ion exchange<br />
Temperature<br />
The mass transfer component <strong>of</strong> the Van Deemter equation can<br />
<strong>of</strong>ten be improved through temperature control. The mass transfer<br />
can be increased by increasing the temperature at which the separation<br />
takes place. The way in which column temperature is<br />
achieved is also <strong>of</strong> significant importance.<br />
Figure 42 shows the effect that increasing column temperature<br />
has on efficiency. It is important to note the role <strong>of</strong> preheating the<br />
mobile phase on the separation. For example, heating only the<br />
column itself and not heating the solvent supplied to it can actually<br />
increase band spreading and reduce column efficiency as shown<br />
in Figure 42. Heating the solvent prior to its arrival at the column<br />
prevents this band spreading and improves the mass transfer associated<br />
with the ion exchange mechanism. The result is an increase<br />
in the column efficiency for any given flow rate.<br />
Figure 42: Effect <strong>of</strong> T on efficiency in ion exchange