DEDICATED TO PROFESSOR HC BROWN ON HIS ... - Sigma-Aldrich
DEDICATED TO PROFESSOR HC BROWN ON HIS ... - Sigma-Aldrich
DEDICATED TO PROFESSOR HC BROWN ON HIS ... - Sigma-Aldrich
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
functionalized, and because the sulfonyl<br />
group can be easily cleaved without causing<br />
any racemization of the chiral center. Very<br />
recently, we prepared optically active<br />
β-hydroxy sulfones in 97−99% ee by the<br />
catalytic reduction of the corresponding<br />
β-keto sulfones using 11b as the catalyst and<br />
BACH-EI TM as the borane source (eq 23). 62<br />
The reduction is also very effective for<br />
hindered aliphatic analogs. 62<br />
4. Asymmetric Reduction of<br />
Prochiral Ketimines<br />
In contrast to the large number of<br />
12 VOL. 35, NO. 1 • 2002<br />
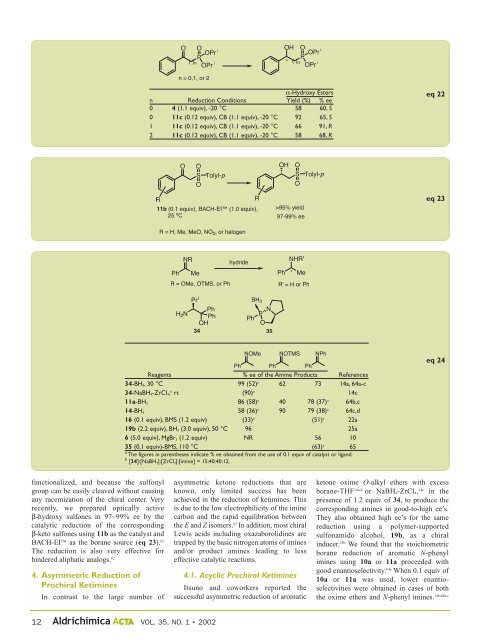
α-Hydroxy Esters<br />
n Reduction Conditions Yield (%) % ee<br />
0 4 (1.1 equiv), -20 °C 58 60, S<br />
0 11c (0.12 equiv), CB (1.1 equiv), -20 °C 92 65, S<br />
1 11c (0.12 equiv), CB (1.1 equiv), -20 °C 66 91, R<br />
2 11c (0.12 equiv), CB (1.1 equiv), -20 °C 58 68, R<br />
Reagents % ee of the Amine Products References<br />
34-BH3, 30 °C 99 (52) a 62 73 14a, 64a-c<br />
34-NaBH4-ZrCl4, b rt (90) a 14c<br />
11a-BH3 86 (58) a 40 78 (37) a 64b,c<br />
14-BH3 58 (36) a 90 79 (38) a 64c,d<br />
16 (0.1 equiv), BMS (1.2 equiv) (33) a (51) a 22a<br />
19b (2.2 equiv), BH3 (3.0 equiv), 50 °C 96 25a<br />
6 (5.0 equiv), MgBr2 (1.2 equiv) NR 56 10<br />
35 (0.1 equiv)-BMS, 110 °C (63) a 65<br />
a The figures in parentheses indicate % ee obtained from the use of 0.1 equiv of catalyst or ligand.<br />
b [34]:[NaBH4]:[ZrCl4]:[imine] = 15:40:40:12.<br />
asymmetric ketone reductions that are<br />
known, only limited success has been<br />
achieved in the reduction of ketimines. This<br />
is due to the low electrophilicity of the imine<br />
carbon and the rapid equilibration between<br />
the E and Z isomers. 63 In addition, most chiral<br />
Lewis acids including oxazaborolidines are<br />
trapped by the basic nitrogen atoms of imines<br />
and/or product amines leading to less<br />
effective catalytic reactions.<br />
4.1. Acyclic Prochiral Ketimines<br />
Itsuno and coworkers reported the<br />
successful asymmetric reduction of aromatic<br />
eq 22<br />
eq 23<br />
eq 24<br />
ketone oxime O-alkyl ethers with excess<br />
borane-THF 14a,b or NaBH4-ZrCl4, 14c in the<br />
presence of 1.2 equiv of 34, to produce the<br />
corresponding amines in good-to-high ee’s.<br />
They also obtained high ee’s for the same<br />
reduction using a polymer-supported<br />
sulfonamido alcohol, 19b, as a chiral<br />
inducer. 25b We found that the stoichiometric<br />
borane reduction of aromatic N-phenyl<br />
imines using 10a or 11a proceeded with<br />
good enantioselectivity. 64a When 0.1 equiv of<br />
10a or 11a was used, lower enantioselectivities<br />
were obtained in cases of both<br />
the oxime ethers and N-phenyl imines. 14b,64b,c