DEDICATED TO PROFESSOR HC BROWN ON HIS ... - Sigma-Aldrich
DEDICATED TO PROFESSOR HC BROWN ON HIS ... - Sigma-Aldrich
DEDICATED TO PROFESSOR HC BROWN ON HIS ... - Sigma-Aldrich
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
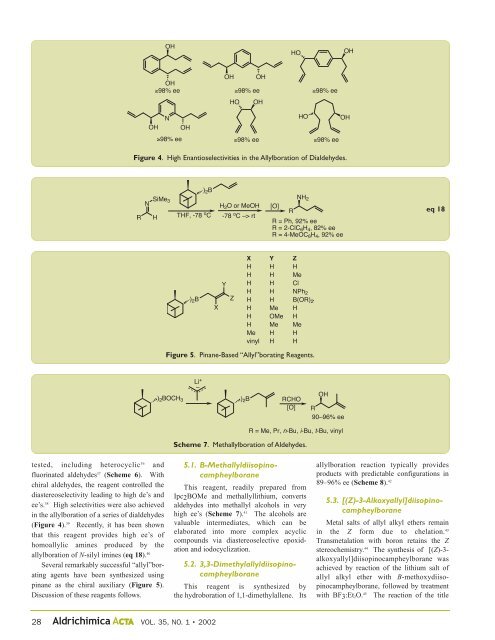
tested, including heterocyclic 36 and<br />
fluorinated aldehydes 37 (Scheme 6). With<br />
chiral aldehydes, the reagent controlled the<br />
diastereoselectivity leading to high de’s and<br />
ee’s. 38 High selectivities were also achieved<br />
in the allylboration of a series of dialdehydes<br />
(Figure 4). 39 Recently, it has been shown<br />
that this reagent provides high ee’s of<br />
homoallylic amines produced by the<br />
allylboration of N-silyl imines (eq 18). 40<br />
Several remarkably successful “allyl”borating<br />
agents have been synthesized using<br />
pinane as the chiral auxiliary (Figure 5).<br />
Discussion of these reagents follows.<br />
28 VOL. 35, NO. 1 • 2002<br />
Figure 4. High Enantioselectivities in the Allylboration of Dialdehydes.<br />
Figure 5. Pinane-Based “Allyl”borating Reagents.<br />
Scheme 7. Methallylboration of Aldehydes.<br />
5.1. B-Methallyldiisopinocampheylborane<br />
This reagent, readily prepared from<br />
Ipc2BOMe and methallyllithium, converts<br />
aldehydes into methallyl alcohols in very<br />
high ee’s (Scheme 7). 41 The alcohols are<br />
valuable intermediates, which can be<br />
elaborated into more complex acyclic<br />
compounds via diastereoselective epoxidation<br />
and iodocyclization.<br />
5.2. 3,3-Dimethylallyldiisopinocampheylborane<br />
This reagent is synthesized by<br />
the hydroboration of 1,1-dimethylallene. Its<br />
eq 18<br />
allylboration reaction typically provides<br />
products with predictable configurations in<br />
89–96% ee (Scheme 8). 42<br />
5.3. [(Z)-3-Alkoxyallyl]diisopinocampheylborane<br />
Metal salts of allyl alkyl ethers remain<br />
in the Z form due to chelation. 43<br />
Transmetalation with boron retains the Z<br />
stereochemistry. 44 The synthesis of [(Z)-3alkoxyallyl]diisopinocampheylborane<br />
was<br />
achieved by reaction of the lithium salt of<br />
allyl alkyl ether with B-methoxydiisopinocampheylborane,<br />
followed by treatment<br />
with BF3:Et2O. 45 The reaction of the title