DEDICATED TO PROFESSOR HC BROWN ON HIS ... - Sigma-Aldrich
DEDICATED TO PROFESSOR HC BROWN ON HIS ... - Sigma-Aldrich
DEDICATED TO PROFESSOR HC BROWN ON HIS ... - Sigma-Aldrich
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
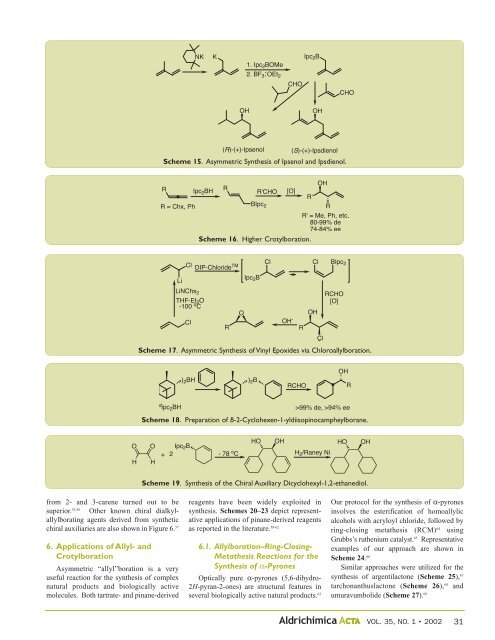
from 2- and 3-carene turned out to be<br />
superior. 55,56 Other known chiral dialkylallylborating<br />
agents derived from synthetic<br />
chiral auxiliaries are also shown in Figure 6. 57<br />
6. Applications of Allyl- and<br />
Crotylboration<br />
Asymmetric “allyl”boration is a very<br />
useful reaction for the synthesis of complex<br />
natural products and biologically active<br />
molecules. Both tartrate- and pinane-derived<br />
Scheme 15. Asymmetric Synthesis of Ipsenol and Ipsdienol.<br />
Scheme 16. Higher Crotylboration.<br />
Scheme 17. Asymmetric Synthesis of Vinyl Epoxides via Chloroallylboration.<br />
Scheme 18. Preparation of B-2-Cyclohexen-1-yldiisopinocampheylborane.<br />
Scheme 19. Synthesis of the Chiral Auxiliary Dicyclohexyl-1,2-ethanediol.<br />
reagents have been widely exploited in<br />
synthesis. Schemes 20–23 depict representative<br />
applications of pinane-derived reagents<br />
as reported in the literature. 58-62<br />
6.1. Allylboration–Ring-Closing-<br />
Metathesis Reactions for the<br />
Synthesis of α-Pyrones<br />
Optically pure α-pyrones (5,6-dihydro-<br />
2H-pyran-2-ones) are structural features in<br />
several biologically active natural products. 63<br />
Our protocol for the synthesis of α-pyrones<br />
involves the esterification of homoallylic<br />
alcohols with acryloyl chloride, followed by<br />
ring-closing metathesis (RCM) 64 using<br />
Grubbs’s ruthenium catalyst. 65 Representative<br />
examples of our approach are shown in<br />
Scheme 24. 66<br />
Similar approaches were utilized for the<br />
synthesis of argentilactone (Scheme 25), 67<br />
tarchonanthuslactone (Scheme 26), 68 and<br />
umuravumbolide (Scheme 27). 69<br />
VOL. 35, NO. 1 • 2002<br />
31