Belay Zeleke Dilnesa - Eawag-Empa Library
Belay Zeleke Dilnesa - Eawag-Empa Library
Belay Zeleke Dilnesa - Eawag-Empa Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
CHAPTER 3 SYNTHETIC FE-CONTAINING HYDRATES<br />
Using the compositions determined from XRD, the solubility products of both<br />
hydroandradite phases were calculated according to the following equations:<br />
KS0 (Ca3Fe2(SiO4)0.95(2)(OH)8.20(2)) = {Ca 2+ } 3 . {FeO2 - } 2 {HSiO3 - } 0.95 . {OH - } 3.05 . {H2O} 2.1<br />
KS0 (Ca3Fe2(SiO4)1.52(2)(OH)5.92(4)) = {Ca 2+ } 3 . {FeO2 - } 2 {HSiO3 - } 1.52 . {OH - } 2.48 . {H2O} 0.96<br />
The calculated values from the dissolution experiments are given in Table 31. The<br />
resulting solubility products are log Ks0= -32.34 and –34.50 at 20 °C and log Ks0 = -33.68<br />
and -35.76 at 50 °C for C3FS0.95H4.1 and C3FS1.52H2.26, respectively. The solubility<br />
products of Fe-Si-hydrogarnet synthesized at 20 °C and equilibrated under oversaturation<br />
are very similar (log Ks0 = -32.98 and –34.63 at 20 °C for C3FS0.95H4.1 and C3FS1.52H2.96).<br />
The values are 7 log units lower than those of the Al-containing siliceous hydrogarnet<br />
determined in this study. Hence, hydroandradites are significantly more stable than the Al<br />
containing hydrogrossulars.<br />
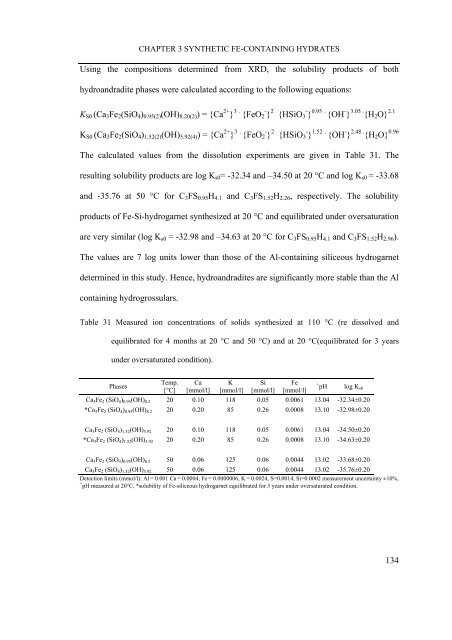
Table 31 Measured ion concentrations of solids synthesized at 110 °C (re dissolved and<br />
equilibrated for 4 months at 20 °C and 50 °C) and at 20 °C(equilibrated for 3 years<br />
under oversaturated condition).<br />
Phases<br />
Temp.<br />
[°C]<br />
Ca<br />
[mmol/l]<br />
K<br />
[mmol/l]<br />
Si<br />
[mmol/l]<br />
Fe<br />
[mmol/l]<br />
+ pH log Ks0<br />
Ca3Fe2 (SiO4)0.95(OH)8.2 20 0.10 118 0.05 0.0061 13.04 -32.34±0.20<br />
*Ca3Fe2 (SiO4) 0.95(OH) 8.2 20 0.20 85 0.26 0.0008 13.10 -32.98±0.20<br />
Ca3Fe2 (SiO4)1.52(OH)5.92 20 0.10 118 0.05 0.0061 13.04 -34.50±0.20<br />
*Ca3Fe2 (SiO4)1.52(OH)5.92 20 0.20 85 0.26 0.0008 13.10 -34.63±0.20<br />
Ca3Fe2 (SiO4)0.95(OH)8.2 50 0.06 125 0.06 0.0044 13.02 -33.68±0.20<br />
Ca3Fe2 (SiO4) 1.52(OH) 5.92 50 0.06 125 0.06 0.0044 13.02 -35.76±0.20<br />
Detection limits (mmol/l): Al = 0.001 Ca = 0.0004, Fe = 0.0000006, K = 0.0024, S=0.0014, Si=0.0002 measurement uncertainty ±10%,<br />
+<br />
pH measured at 20°C, *solubility of Fe-siliceous hydrogarnet equilibrated for 3 years under oversaturated condition.<br />
134