Belay Zeleke Dilnesa - Eawag-Empa Library
Belay Zeleke Dilnesa - Eawag-Empa Library
Belay Zeleke Dilnesa - Eawag-Empa Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
CHAPTER 2 MATERIALS AND METHODS<br />
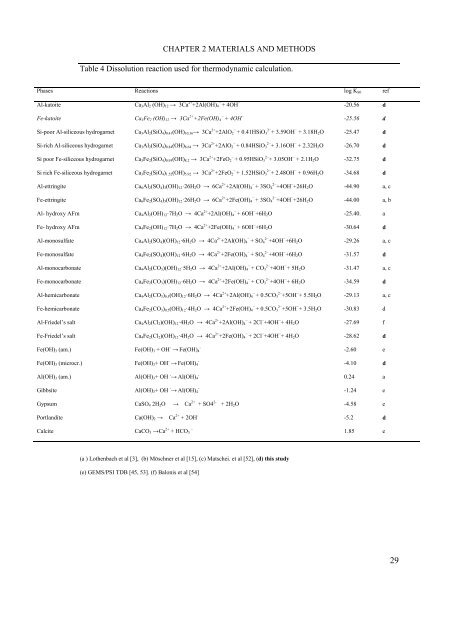
Table 4 Dissolution reaction used for thermodynamic calculation.<br />
Phases Reactions log KS0 ref<br />
Al-katoite Ca3Al2 (OH)12 → 3Ca 2+ +2Al(OH)4 − + 4OH − -20.56 d<br />
Fe-katoite Ca3Fe2 (OH)12 → 3Ca 2+ +2Fe(OH)4 − + 4OH − -25.56 d<br />
Si-poor Al-siliceous hydrogarnet Ca3Al2(SiO4)0.41(OH)10.36→ 3Ca 2+ +2AlO2 − + 0.41HSiO3 2− + 3.59OH − + 3.18H2O -25.47 d<br />
Si-rich Al-siliceous hydrogarnet Ca3Al2(SiO4)0.84(OH)8.64 → 3Ca 2+ +2AlO2 − + 0.84HSiO3 2− + 3.16OH − + 2.32H2O -26.70 d<br />
Si poor Fe-siliceous hydrogarnet Ca3Fe2(SiO4)0.95(OH)8.2 → 3Ca 2+ +2FeO2 − + 0.95HSiO3 2− + 3.05OH − + 2.1H2O -32.75 d<br />
Si rich Fe-siliceous hydrogarnet Ca3Fe2(SiO4)1.52(OH)5.92 → 3Ca 2+ +2FeO2 − + 1.52HSiO3 2− + 2.48OH − + 0.96H2O -34.68 d<br />
Al-ettringite Ca6Al2(SO4)3(OH)12·26H2O → 6Ca 2+ +2Al(OH)4 − + 3SO4 2− +4OH − +26H2O -44.90 a, c<br />
Fe-ettringite Ca6Fe2(SO4)3(OH)12·26H2O → 6Ca 2+ +2Fe(OH)4 − + 3SO4 2− +4OH − +26H2O -44.00 a, b<br />
Al- hydroxy AFm Ca4Al2(OH)12·7H2O → 4Ca 2+ +2Al(OH)4 − + 6OH − +6H2O -25.40. a<br />
Fe- hydroxy AFm Ca4Fe2(OH)12·7H2O → 4Ca 2+ +2Fe(OH)4 − + 6OH − +6H2O -30.64 d<br />
Al-monosulfate Ca4Al2(SO4)(OH)12·6H2O → 4Ca 2+ +2Al(OH)4 − + SO4 2− +4OH − +6H2O -29.26 a, c<br />
Fe-monosulfate Ca4Fe2(SO4)(OH)12·6H2O → 4Ca 2+ +2Fe(OH)4 − + SO4 2− +4OH − +6H2O -31.57 d<br />
Al-monocarbonate Ca4Al2(CO3)(OH)12·5H2O → 4Ca 2+ +2Al(OH)4 − + CO3 2− +4OH − + 5H2O -31.47 a, c<br />
Fe-monocarbonate Ca4Fe2(CO3)(OH)12·6H2O → 4Ca 2+ +2Fe(OH)4 − + CO3 2− +4OH − + 6H2O -34.59 d<br />
Al-hemicarbonate Ca4Al2(CO3)0.5(OH)12·6H2O → 4Ca 2+ +2Al(OH)4 − + 0.5CO3 2− +5OH − + 5.5H2O -29.13 a, c<br />
Fe-hemicarbonate Ca4Fe2(CO3)0.5(OH)12·4H2O → 4Ca 2+ +2Fe(OH)4 − + 0.5CO3 2− +5OH − + 3.5H2O -30.83 d<br />
Al-Friedel’s salt Ca4Al2(Cl2)(OH)12·4H2O → 4Ca 2+ +2Al(OH)4 − + 2Cl − +4OH − + 4H2O -27.69 f<br />
Fe-Friedel’s salt Ca4Fe2(Cl2)(OH)12·4H2O → 4Ca 2+ +2Fe(OH)4 − + 2Cl − +4OH − + 4H2O -28.62 d<br />
Fe(OH)3 (am.) Fe(OH)3 + OH - → Fe(OH)4 - -2.60 e<br />
Fe(OH)3 (microcr.) Fe(OH)3+ OH - → Fe(OH)4 - -4.10 d<br />
Al(OH)3 (am.) Al(OH)3+ OH - → Al(OH)4 - 0.24 a<br />
Gibbsite Al(OH)3+ OH - → Al(OH)4 - -1.24 e<br />
Gypsum CaSO4 2H2O → Ca 2+ + SO4 2- + 2H2O -4.58 e<br />
Portlandite Ca(OH)2 → Ca 2+ + 2OH - -5.2 d<br />
Calcite CaCO3 →Ca 2+ + HCO3 − 1.85 e<br />
(a ) Lothenbach et al [3], (b) Möschner et al [15], (c) Matschei. et al [52], (d) this study<br />
(e) GEMS/PSI TDB [45, 53]. (f) Balonis et al [54]<br />
29