- Page 1:
Fe-containing hydrates and their fa
- Page 4 and 5:
The measured composition of the liq
- Page 6 and 7:
ZUSAMMENFASSUNG Thermodynamische Mo
- Page 8 and 9:
konnten die Fe-haltigen Hydrate im
- Page 10 and 11:
I owe to thank all the laboratory t
- Page 12 and 13:
Table 19 Compositions of Al/Fe-mono
- Page 14 and 15:
LIST OF FIGURES Fig. 1 Calculated v
- Page 16 and 17:
Fig. 34 Calculated solubility produ
- Page 18 and 19:
Fig. 62 Calculated solubility produ
- Page 20 and 21:
Table of Contents TABLE OF CONTENTS
- Page 22 and 23:
TABLE OF CONTENTS 3. SYNTHETIC FE
- Page 24 and 25:
TABLE OF CONTENTS 3.5.4. Solid solu
- Page 26 and 27:
1 INTRODUCTION CHAPTER 1 INTRODUCTI
- Page 28 and 29:
CHAPTER 1 INTRODUCTION be formed. T
- Page 30 and 31:
cm 3 /100 g cement 85 80 75 70 65 6
- Page 32 and 33:
CHAPTER 1 INTRODUCTION 1.4 Characte
- Page 34 and 35: CHAPTER 1 INTRODUCTION Fig. 4 Sampl
- Page 36 and 37: CHAPTER 1 INTRODUCTION Identificat
- Page 38 and 39: CHAPTER 2 MATERIALS AND METHODS han
- Page 40 and 41: 3CaOAl2O3 + 6H2O → 3CaOAl2O36H2O
- Page 42 and 43: CHAPTER 2 MATERIALS AND METHODS Tab
- Page 44 and 45: CHAPTER 2 MATERIALS AND METHODS 2.2
- Page 46 and 47: CHAPTER 2 MATERIALS AND METHODS fil
- Page 48 and 49: CHAPTER 2 MATERIALS AND METHODS at
- Page 50 and 51: CHAPTER 2 MATERIALS AND METHODS the
- Page 52 and 53: CHAPTER 2 MATERIALS AND METHODS 0 t
- Page 54 and 55: CHAPTER 2 MATERIALS AND METHODS Tab
- Page 56 and 57: CHAPTER 2 MATERIALS AND METHODS 2.3
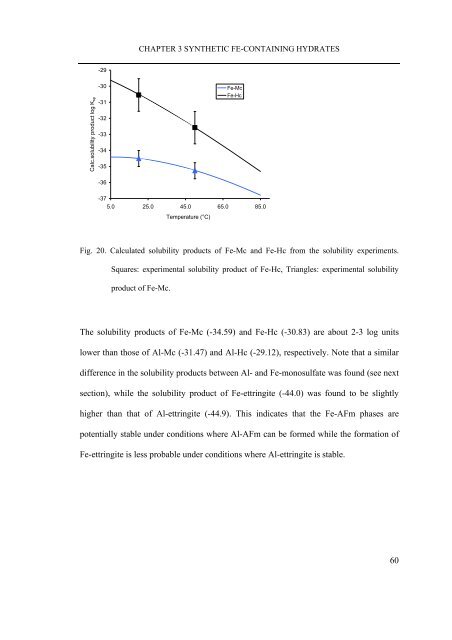
- Page 58 and 59: CHAPTER 2 MATERIALS AND METHODS The
- Page 60 and 61: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 62 and 63: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 64 and 65: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 66 and 67: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 68 and 69: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 70 and 71: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 72 and 73: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 74 and 75: a). b). CHAPTER 3 SYNTHETIC FE-CONT
- Page 76 and 77: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 78 and 79: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 80 and 81: o layer thickness (A) 8.0 7.8 7.6 C
- Page 82 and 83: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 86 and 87: Phase log KSo CHAPTER 3 SYNTHETIC F
- Page 88 and 89: volume [cm 3 ] 700 600 500 400 300
- Page 90 and 91: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 92 and 93: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 94 and 95: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 96 and 97: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 98 and 99: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 100 and 101: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 102 and 103: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 104 and 105: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 106 and 107: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 108 and 109: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 110 and 111: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 112 and 113: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 114 and 115: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 116 and 117: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 118 and 119: Intensity [arb. units] Fe-Fr CHAPTE
- Page 120 and 121: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 122 and 123: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 124 and 125: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 126 and 127: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 128 and 129: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 130 and 131: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 132 and 133: CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 134 and 135:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 136 and 137:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 138 and 139:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 140 and 141:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 142 and 143:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 144 and 145:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 146 and 147:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 148 and 149:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 150 and 151:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 152 and 153:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 154 and 155:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 156 and 157:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 158 and 159:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 160 and 161:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 162 and 163:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 164 and 165:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 166 and 167:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 168 and 169:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 170 and 171:
CHAPTER 3 SYNTHETIC FE-CONTAINING H
- Page 172 and 173:
CHAPTER 4 FE-CONTAINING HYDRATES IN
- Page 174 and 175:
CHAPTER 4 FE-CONTAINING HYDRATES IN
- Page 176 and 177:
CHAPTER 4 FE-CONTAINING HYDRATES IN
- Page 178 and 179:
CHAPTER 4 FE-CONTAINING HYDRATES IN
- Page 180 and 181:
CHAPTER 4 FE-CONTAINING HYDRATES IN
- Page 182 and 183:
(Al+Fe)/Ca (Al+Fe)/Ca 0.8 0.6 0.4 0
- Page 184 and 185:
CHAPTER 4 FE-CONTAINING HYDRATES IN
- Page 186 and 187:
CHAPTER 4 FE-CONTAINING HYDRATES IN
- Page 188 and 189:
k 3 (k) CHAPTER 4 FE-CONTAINING HYD
- Page 190 and 191:
CHAPTER 4 FE-CONTAINING HYDRATES IN
- Page 192 and 193:
CHAPTER 4 FE-CONTAINING HYDRATES IN
- Page 194 and 195:
CHAPTER 4 FE-CONTAINING HYDRATES IN
- Page 196 and 197:
CHAPTER 4 FE-CONTAINING HYDRATES IN
- Page 198 and 199:
CHAPTER 4 FE-CONTAINING HYDRATES IN
- Page 200 and 201:
differentiated relative weight Weig
- Page 202 and 203:
Early reaction CHAPTER 4 FE-CONTAIN
- Page 204 and 205:
weight loss in % differentiated rel
- Page 206 and 207:
CHAPTER 4 FE-CONTAINING HYDRATES IN
- Page 208 and 209:
5. GENERAL CONCLUSION AND OUTLOOK a
- Page 210 and 211:
Speciation of iron(III) in hydrated
- Page 212 and 213:
5. GENERAL CONCLUSION AND OUTLOOK
- Page 214 and 215:
Techniques XRD X-ray diffraction TG
- Page 216 and 217:
APPENDIX APPENDIX Appendix A: Addit
- Page 218 and 219:
Intensity (arb. Units) P Fe 2O 3 R
- Page 220 and 221:
APPENDIX Appendix B7 XRD patterns o
- Page 222 and 223:
REFERENCES REFRENCES [1] H.F.W. Tay
- Page 224 and 225:
REFRENCES [29] M. Vespa, R. Dähn,
- Page 226 and 227:
REFRENCES [54] M. Balonis, B. Lothe
- Page 228 and 229:
REFRENCES [81] H.F.W. Taylor, Cryst
- Page 230 and 231:
REFRENCES [107] K. Kyritsis, N. Mel
- Page 232 and 233:
REFRENCES [134] M. Wilke, F. Farges
- Page 235 and 236:
CURRCULUM VITAE Curriculum Vitae Na