ER/PR pharmDx™ Interpretation Manual - Dako
ER/PR pharmDx™ Interpretation Manual - Dako
ER/PR pharmDx™ Interpretation Manual - Dako
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>ER</strong>/<strong>PR</strong> pharmDx tM overview<br />
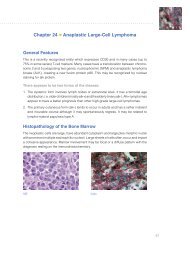
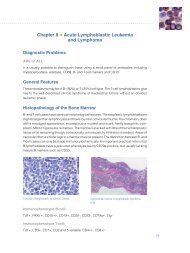
the <strong>ER</strong>/<strong>PR</strong> pharmDx tM Kit is a semi-quantitative<br />
immunohistochemical (IHC) assay to identify estrogen<br />
receptor (<strong>ER</strong>) and progesterone receptor (<strong>PR</strong>) expression<br />
in normal and neoplastic tissues, formalin-fixed and<br />
paraffin-embedded for histological evaluation. <strong>ER</strong>/<strong>PR</strong><br />
pharmDx tM specifically detects the <strong>ER</strong> alpha protein as<br />
well as the <strong>PR</strong> protein located in the nuclei of <strong>ER</strong> and<br />
<strong>PR</strong>-expressing cells, respectively.<br />
Investigations into the biological mechanisms for breast<br />
cancer have found that the growth rate is dependent on<br />
the presence of estrogen or progesterone or both in most<br />
breast cancers. thus, estrogen receptor and proges-<br />
terone receptor status in breast cancer is considered to<br />
be a validated prognostic and predictive factor for patient<br />
management for anti-hormonal therapy. 1-5<br />
<strong>ER</strong>/<strong>PR</strong> pharmDx tM is indicated as an aid in identifying<br />
patients eligible for treatment with anti-hormonal or<br />
aromatase inhibitor therapies, as well as an aid in the<br />
prognosis and management of breast cancer.<br />
<strong>ER</strong>/<strong>PR</strong> pharmDx Provides the Basis<br />
for Reliable <strong>ER</strong> and <strong>PR</strong> Assessment<br />
n FDA 510(k) cleared, standard, reproducible assay.<br />
n new, highly specific <strong>ER</strong> antibody cocktail and <strong>PR</strong><br />
antibody with demonstrated sensitivity and specificity.<br />
n optimized protocol with clinically validated scoring<br />
system for the determination of <strong>ER</strong>/<strong>PR</strong> status<br />
applicable in the management of breast cancer<br />
patients. 5-9<br />
n Concordance demonstrated between <strong>ER</strong>/<strong>PR</strong><br />
pharmDx tM and an established reference method<br />
with positive/negative cut-off IHC score calibrated<br />
using samples with known biochemical and clinical<br />
response data. 10<br />
n Verified cut-off IHC score for positivity for<br />
<strong>ER</strong>/<strong>PR</strong> pharmDx tM .<br />
n FDA clearance and confidence in test sensitivity and<br />
specificity lessens the burden of extensive validation<br />
by laboratory staff.<br />
<strong>ER</strong>/<strong>PR</strong> pharmDx <strong>Interpretation</strong> <strong>Manual</strong><br />
oV<strong>ER</strong>VIEw<br />
3