smallpox vaccine and vaccination in the intensified ... - libdoc.who.int
smallpox vaccine and vaccination in the intensified ... - libdoc.who.int
smallpox vaccine and vaccination in the intensified ... - libdoc.who.int
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
11. VACCINATION IN THE INTENSIFIED PROGRAMME 559<br />
Table I 1.10. Variation <strong>in</strong> results of titrations of vacc<strong>in</strong>ia virus accord<strong>in</strong>g to age of chick embryos at time of<br />
<strong>in</strong>oculations<br />
Length of<br />
Sample studied<br />
<strong>in</strong>cubation<br />
-I -L,-#.<br />
"I LIIICK<br />
A B C D E<br />
Average<br />
embryo (days' Titreb DlfferenceC Tltreb DlfferenceC Tltreb Dlfferencec Titreb Differencec Titreb Differencec difference<br />
aSource: A. Bernste<strong>in</strong> (personal communication, 1969).<br />
Expressed as loglo pock-form<strong>in</strong>g units per ml.<br />
C From <strong>the</strong> titre obta<strong>in</strong>ed with i2day-old chlck embryos.<br />
f<strong>in</strong>d<strong>in</strong>gs, an <strong>in</strong>oculum of 0.1 m1 <strong>and</strong> an <strong>in</strong>- Moisture content<br />
cubati& period of 12 days for <strong>the</strong> eggs were<br />
acce~ted as st<strong>and</strong>ard.<br />
Although residual moisture appeared to be<br />
It was also suggested that <strong>the</strong> quality of <strong>the</strong><br />
an important factor <strong>in</strong>fluenc<strong>in</strong>g <strong>the</strong> stability<br />
eggs from different geographical areas might<br />
of freeze-dried <strong>vacc<strong>in</strong>e</strong>. it was difficult to<br />
affect <strong>the</strong> assavs. For reasons unknown. vacestablish<br />
criteria for <strong>the</strong> moisture content.<br />
c<strong>in</strong>es assayed <strong>in</strong> develop<strong>in</strong>g countries often<br />
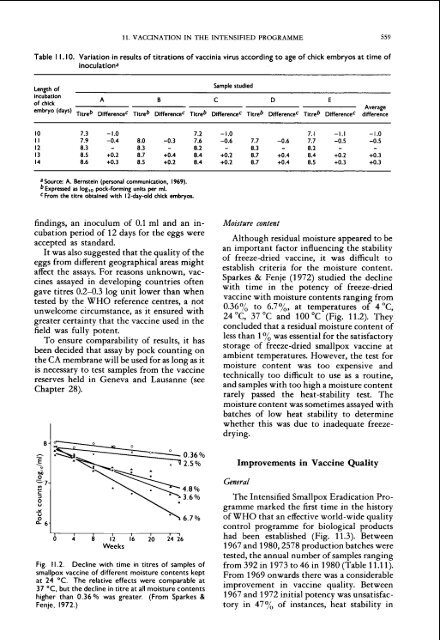
Sparkes & Fenje (1972) studied <strong>the</strong> decl<strong>in</strong>e<br />
gave titres 0.2-0.3 log unit lower than when<br />
with time <strong>in</strong> <strong>the</strong> potency of freeze-dried<br />
tested by <strong>the</strong> WHO reference centres, a not<br />
<strong>vacc<strong>in</strong>e</strong> with moisture contents rang<strong>in</strong>g from<br />
unwelcome circumstance. as it ensured with<br />
0.36% to 6.7%, at temperatures of 4 "C,<br />
greater certa<strong>in</strong>ty that <strong>the</strong> <strong>vacc<strong>in</strong>e</strong> used <strong>in</strong> <strong>the</strong><br />
24 "C, 37 "C <strong>and</strong> 100 "C (Fig. 11.2). They<br />
field was fully potent.<br />
concluded that a residual moisture content of<br />
To ensure c6m~arabilitv of results. it has<br />
less than 1 was essential for <strong>the</strong> satisfactory<br />
been decided that assay by pock count<strong>in</strong>g on<br />
storage of freeze-dried <strong>smallpox</strong> <strong>vacc<strong>in</strong>e</strong> at<br />
<strong>the</strong> CA membrane will be used for as long as it<br />
ambient temperatures. ~owever, <strong>the</strong> test for<br />
is necessary to test samples from <strong>the</strong> <strong>vacc<strong>in</strong>e</strong><br />
moisture content was too expensive <strong>and</strong><br />
reserves held <strong>in</strong> ~eneva <strong>and</strong> Lausanne (see<br />
technically too difficult to use as a rout<strong>in</strong>e,<br />
Chapter 28).<br />
<strong>and</strong> samples with too high a moisture content<br />
rarely passed <strong>the</strong> heat-stabilitv test. The<br />
moisture content was sometimes Hssayed with<br />
batches of low heat stability to determ<strong>in</strong>e<br />
whe<strong>the</strong>r this was due to <strong>in</strong>adequate freezedry<strong>in</strong>g.<br />
L<br />
I 1<br />
0 4 8 12 16 20 2426<br />
Weeks<br />
Fig. 11.2. Decl<strong>in</strong>e with time <strong>in</strong> titres of samples of<br />
<strong>smallpox</strong> <strong>vacc<strong>in</strong>e</strong> of different moisture contents kept<br />
at 24 "C. The relative effects were comparable at<br />
37 "C. but <strong>the</strong> decl<strong>in</strong>e <strong>in</strong> titre at all moisture contents<br />
higher than 0.36% was greater. (From Sparkes &<br />
Fenje, 1972.)<br />
Improvements <strong>in</strong> Vacc<strong>in</strong>e Quality<br />
The Intensified Smallpox Eradication Pro-<br />
gramme marked <strong>the</strong> first time <strong>in</strong> <strong>the</strong> history<br />
of WHO that an effective world-wide quality<br />
control programme for biological products<br />
had been established (Fig. 11.3). Between<br />
1967 <strong>and</strong> 1980,2578 production batches were<br />
tested, <strong>the</strong> annual number of samples rang<strong>in</strong>g<br />
from 392 <strong>in</strong> 1973 to 46 <strong>in</strong> 1980 (Table 11.1 1).<br />
From 1969 onwards <strong>the</strong>re was a considerable<br />
improvement <strong>in</strong> <strong>vacc<strong>in</strong>e</strong> quality. Between<br />
1967 <strong>and</strong> 1972 <strong>in</strong>itial potency was unsatisfac-<br />
tory <strong>in</strong> 47% of <strong>in</strong>stances, heat stability <strong>in</strong>