smallpox vaccine and vaccination in the intensified ... - libdoc.who.int
smallpox vaccine and vaccination in the intensified ... - libdoc.who.int
smallpox vaccine and vaccination in the intensified ... - libdoc.who.int
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
11. VACCINATION IN THE INTENSIFIED PROGRAMME<br />
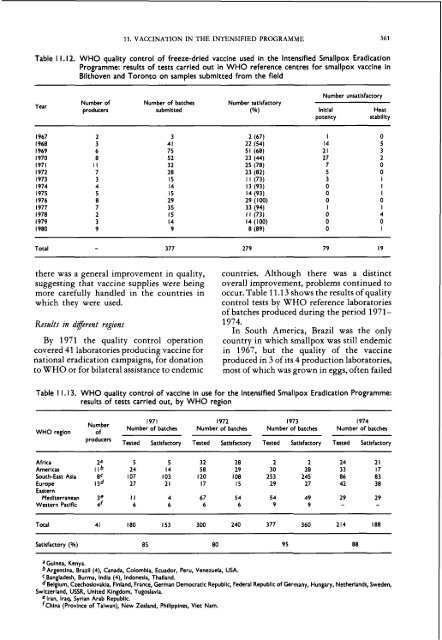
Table 1 1.12. WHO quality control of freeze-dried <strong>vacc<strong>in</strong>e</strong> used <strong>in</strong> <strong>the</strong> lntensified Smallpox Eradication<br />
Programme: results of tests carried out <strong>in</strong> WHO reference centres for <strong>smallpox</strong> <strong>vacc<strong>in</strong>e</strong> <strong>in</strong><br />
Bilthoven <strong>and</strong> Toronto on samples submitted from <strong>the</strong> field<br />
Year<br />
Number unsatlsfactory<br />
Number of Number of batches Number satlsfactory<br />
producers submitted (90) lnitlal Heat<br />
potency stability<br />
Total - 377 279 79 19<br />
<strong>the</strong>re was a general improvement <strong>in</strong> quality, countries. Although <strong>the</strong>re was a dist<strong>in</strong>ct<br />
suggest<strong>in</strong>g that <strong>vacc<strong>in</strong>e</strong> supplies were be<strong>in</strong>g overall improvement, problems cont<strong>in</strong>ued to<br />
more carefully h<strong>and</strong>led <strong>in</strong> <strong>the</strong> countries <strong>in</strong> occur. Table 11.13 shows <strong>the</strong> results of quality<br />
which <strong>the</strong>y were used. control tests by WHO reference laboratories<br />
of batches produced dur<strong>in</strong>g <strong>the</strong> period 1971-<br />
Results <strong>in</strong> dgerent regions<br />
1974.<br />
In South America, Brazil was <strong>the</strong> onlv<br />
By 1971 <strong>the</strong> quality control operation country <strong>in</strong> which <strong>smallpox</strong> was still endemic<br />
covered 41 laboratories produc<strong>in</strong>g <strong>vacc<strong>in</strong>e</strong> for <strong>in</strong> 1967, but <strong>the</strong> quality of <strong>the</strong> <strong>vacc<strong>in</strong>e</strong><br />
national eradication campaigns, for donation produced <strong>in</strong> 3 of its 4 production laboratories,<br />
to WHO or for bilateral assistance to endemic most of which was grown <strong>in</strong> eggs, often failed<br />
Table 1 1.13. WHO quality control of <strong>vacc<strong>in</strong>e</strong> <strong>in</strong> use for <strong>the</strong> lntensified Smallpox Eradication Programme:<br />
results of tests carried out, by WHO region<br />
WHO region<br />
1971 1972 1973 1974<br />
Number<br />
of<br />
Number of batches Number of batches Number of batches Number of batches<br />
Tested Satisfactory Tested Satisfactory Tested Satisfactory Tested Satlsfactory<br />
Africa 2a 5 5 32 28 2 2 24 2 1<br />
Amerlcas I lb 24 14 58 29 30 28 33 17<br />
South-East Asia BC 107 103 120 108 253 245 86 83<br />
Europe 1 3 ~ 27 2 1 17 15 29 27 42 38<br />
Eastern<br />
Medlterranean 3e I I 4 67 54 54 49 29 29<br />
Western Paciflc 4f 6 6 6 6 9 9 - -<br />
Total 41 180 153 300 240 377 360 214 188<br />
htlsfactory (90) 85 80 95 88<br />
a Gu<strong>in</strong>ea, Kenya.<br />
Argent<strong>in</strong>a, Brazil (4), Canada, Colombia, Ecuador, Peru, Venezuela, USA.<br />
C Bangladesh, Burma, Indh (4), Indonesia, Thail<strong>and</strong>.<br />
Belgium, Czechoslovakia, Flnl<strong>and</strong>, France, German Democratlc Republlc, Federal Republlc of Germany, Hungary, Ne<strong>the</strong>rl<strong>and</strong>s, Sweden,<br />
Switzerl<strong>and</strong>, USSR, United K<strong>in</strong>gdom, Yugoslavia.<br />
elran, Iraq, Syrian Arab Republic.<br />
f~h<strong>in</strong>a (Provlnce of Talwan), New Zeal<strong>and</strong>, Philipp<strong>in</strong>es, Vlet Nam.<br />
561